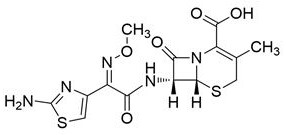
A kind of detection method of cefotaxime sodium related substance
A technology of cefotaxime sodium and detection method, applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problem of inability to separate and detect 12 known impurities in cefotaxime sodium, poor antibacterial activity of Staphylococcus aureus, inability to Quality and other issues, to achieve the effect of convenient quality inspection and monitoring, good specificity and high reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
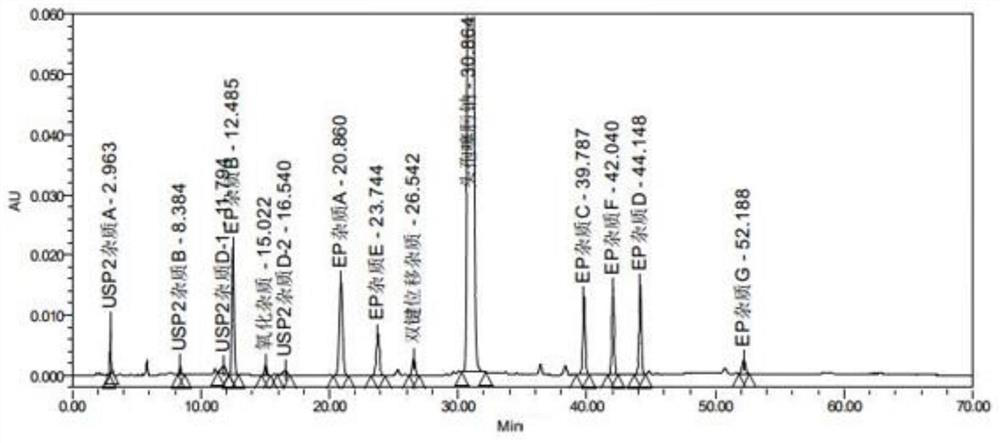
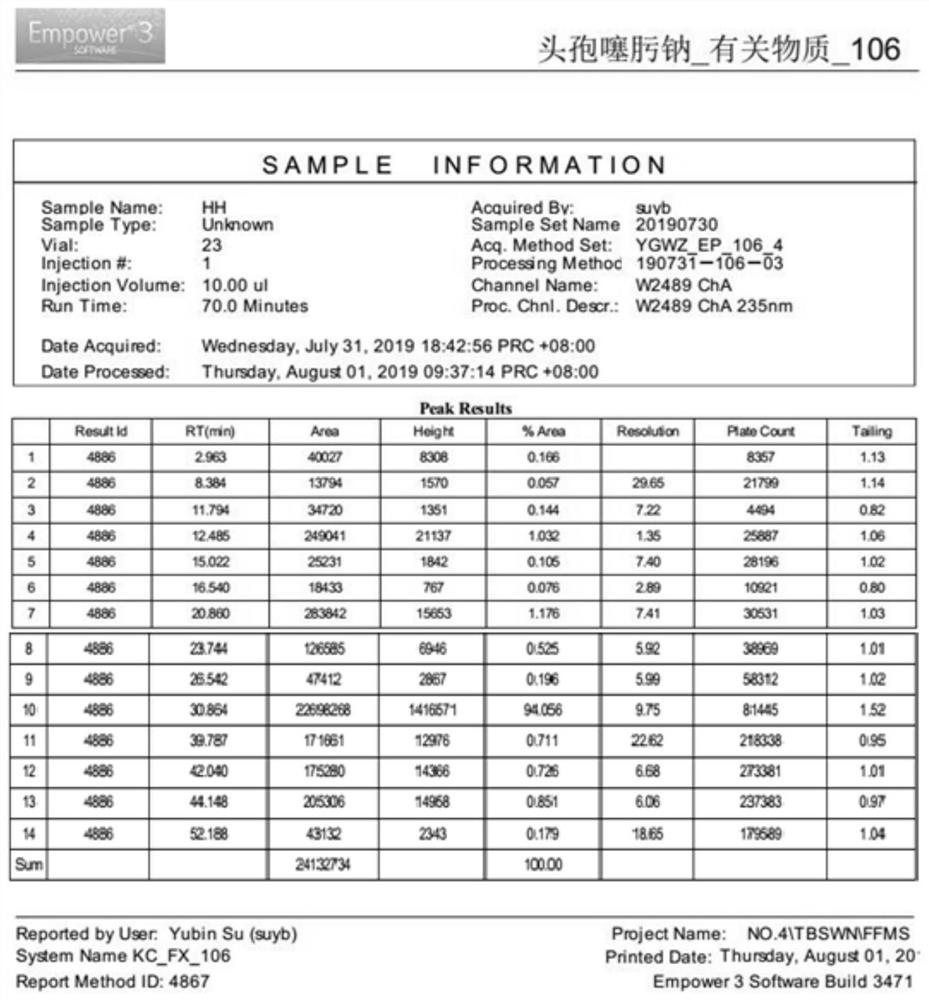
[0058] The present embodiment provides a method for the determination of related substances of cefotaxime sodium by high performance liquid chromatography, which is determined using the following conditions:
[0059] Chromatographic column: Octadecylsilane bonded silica gel is used as a filler, and a chromatographic column with a specification of CAPCELL PAK MGⅡC18 4.6×250mm and 5μm is selected;
[0060] Mobile phase A: phosphate buffer (weigh 7.1 g of anhydrous disodium hydrogen phosphate, add 1000 ml of water to dissolve, adjust the pH value to 6.25 with phosphoric acid);
[0061] Mobile phase B: methanol; adjust the pH to 6.0-6.5 with phosphoric acid;
[0062] Column temperature: 25°C;
[0063] Detection wavelength 1: 235nm;
[0064] Flow rate: 1.0mL / min;
[0065] Solvent: by volume ratio, mobile phase A: mobile phase B (90:10);
[0066] Injection volume: 10μL
[0067] Gradient elution was used.
[0068] The gradient elution procedure is:
[0069] Table 3 Gradient el...
experiment example 1
[0076] Experimental Example 1 System Suitability Test
[0077] Preparation of each impurity positioning solution: Accurately weigh cefotaxime sodium impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, USP-impurity A, USP-impurity B , USP-impurity D and cefotaxime reference substance in appropriate amount, add solvent respectively [in terms of volume ratio, mobile phase A: mobile phase B (90:10)] dissolve and dilute to make about impurity A, B, 10 μg each of C, D, E, and F, 2 μg each of impurity G, impurity H, and impurity I, and 1.5 μg each of impurities USP-A, USP-B, and USP-D, as the positioning solution for each impurity;
[0078] Preparation of the test solution: Get about 25 mg of cefotaxime sodium, accurately weighed, put in a 25 ml measuring bottle, add solvent [in volume ratio, mobile phase A: mobile phase B (90:10)] to dissolve and dilute to the scale, shake well, and you get it;
[0079] Preparation of referen...
experiment example 2
[0086] Experimental example 2 linearity and range test
[0087] Solvent: [by volume ratio, mobile phase A: mobile phase B (90:10)]
[0088] Linear sample solution: Accurately weigh cefotaxime sodium impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, USP-impurity A, USP-impurity B, USP- Add appropriate amount of impurity D and cefotaxime sodium reference substance respectively, add solvent [in terms of volume ratio, mobile phase A: mobile phase B (90:10)] to dissolve and dilute into a series of linear sample solutions, shake well, and get ready.
[0089] Determination: Carry out according to the determination method condition of embodiment 1.
[0090] Precisely measure 10 μL of the above-mentioned solutions, inject them into the liquid chromatograph, and record the chromatograms. The results are shown in Tables 5-6.
[0091] Table 5 Linearity and range test results
[0092]
[0093]
[0094] Table 6 Linearity an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com