Pharmaceutical composition for nasal administration
A pharmaceutical composition and transnasal technology, applied in the directions of drug combination, drug delivery, and pharmaceutical formulation, can solve problems such as failure, achieve high drug efficacy, achieve non-invasive, and low side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0062] Hereinafter, although an Example is shown and this invention is demonstrated more concretely, this invention is not limited to these Examples.
experiment example
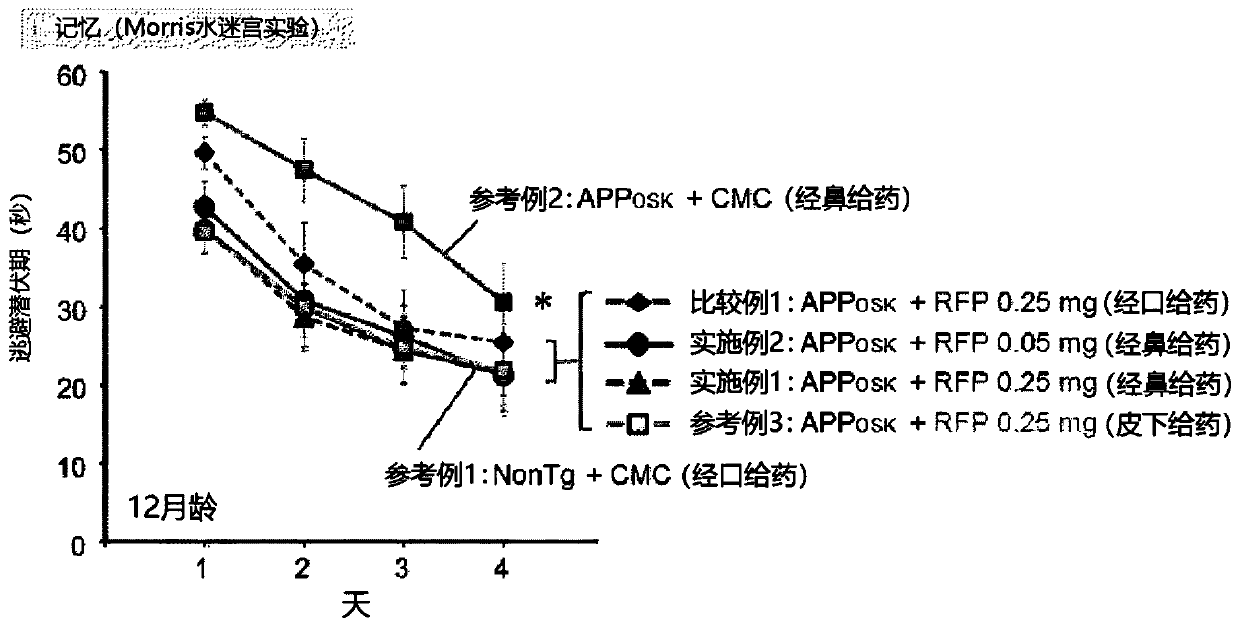
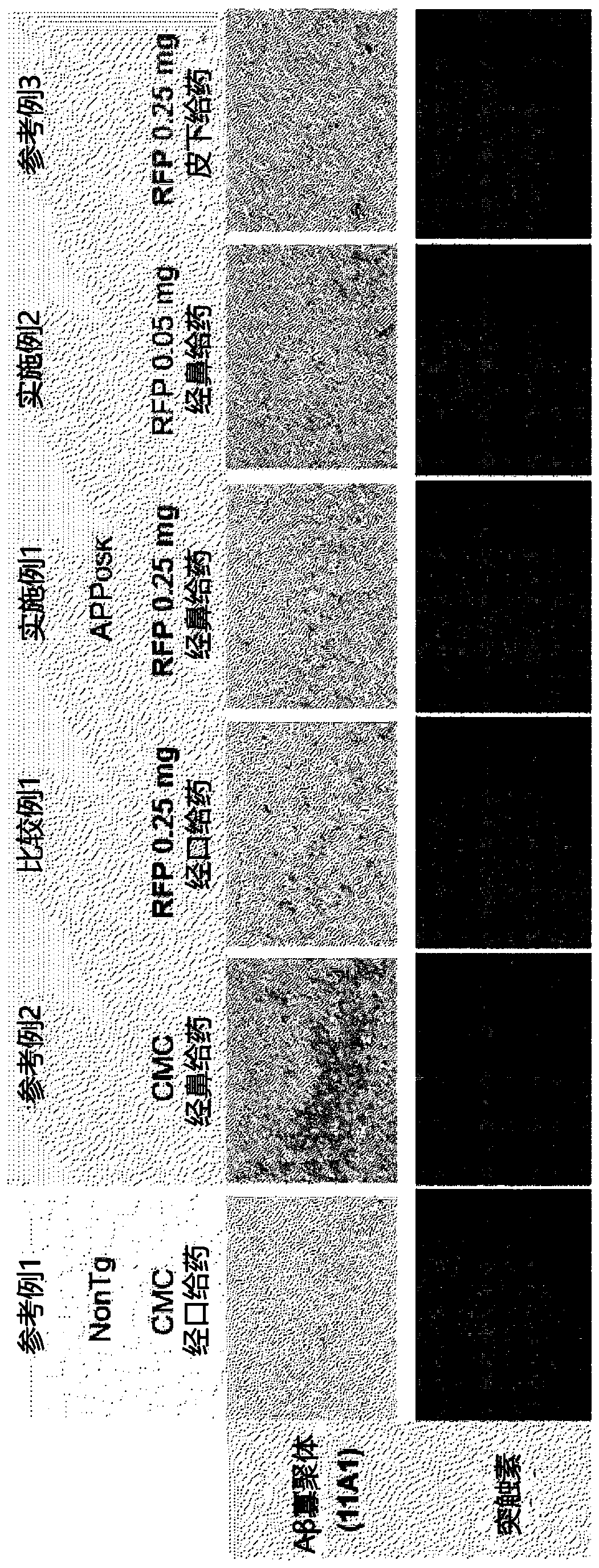
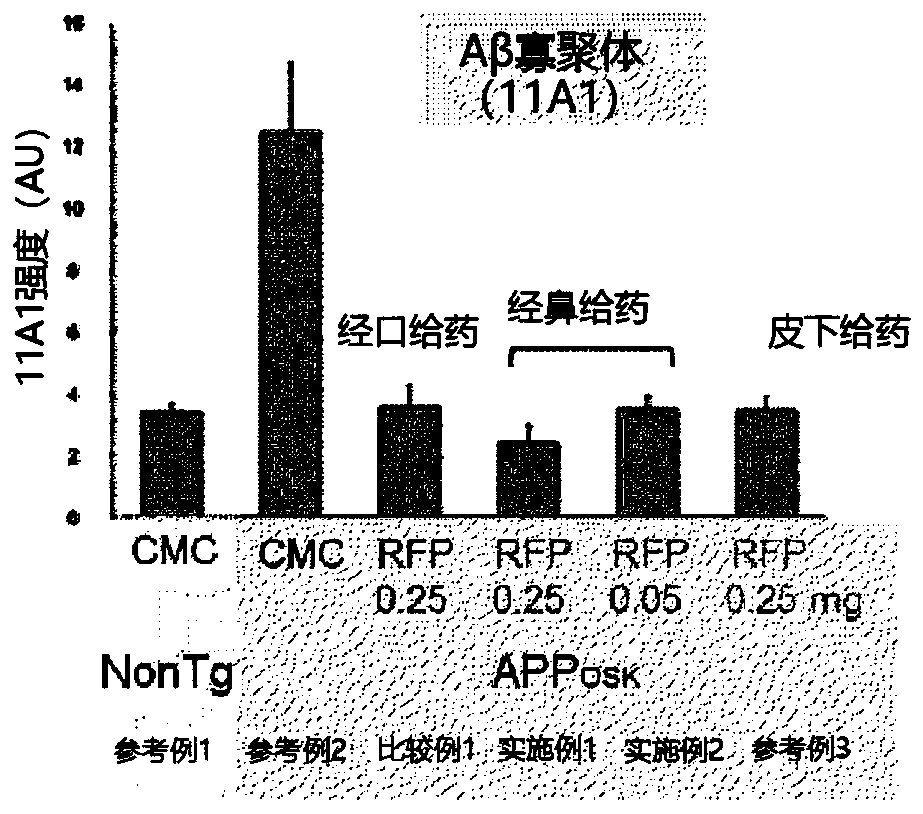
[0064] In this experimental example, the administration composition containing rifampicin or not containing rifampicin was administered daily for one month to the mice shown in Table 1 at the dosage and usage shown in Table 1.
[0065] (administration object)
[0066] Preparation of 11-month-old male APP OSK Mice (Tomiyama et al. J Neurosci. 2010; 30:4845-56). app OSK The body weight of the mice is about 30 g. will APP OSK 60 mice were divided into 5 groups of 12 each. In addition, 12 wild-type mice (non-transgenic littermates (non-Tg littermate)) of the same age were prepared. It should be noted that APP OSK Mice are amyloid precursor protein (APP) transgenic mice (Alzheimer's disease model), showing accumulation of β-amyloid (Aβ).
[0067] (drug composition)
[0068] Rifampicin (RFP; Sigma-Aldrich, Rifampicin≥97% (HPLC), powder, alias: 3-(4-methylpiperazinyliminomethyl) rifamycin SV, rifamycin AMP , rifampicin, R3501) was suspended in 0.5w / v% carboxymethylcellulose ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com