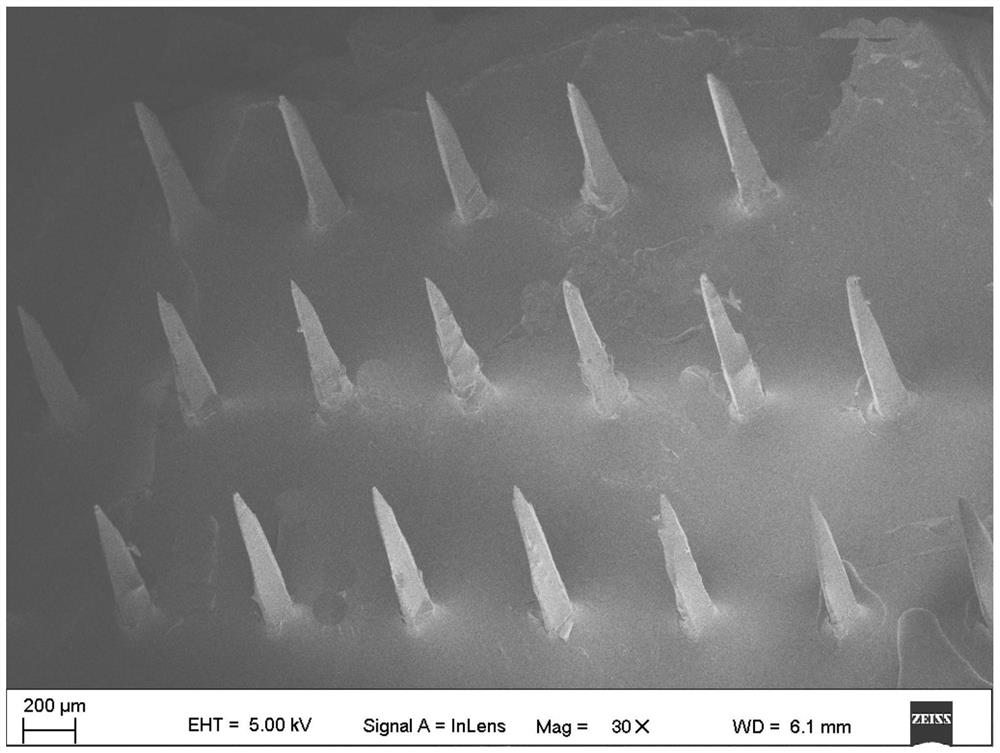
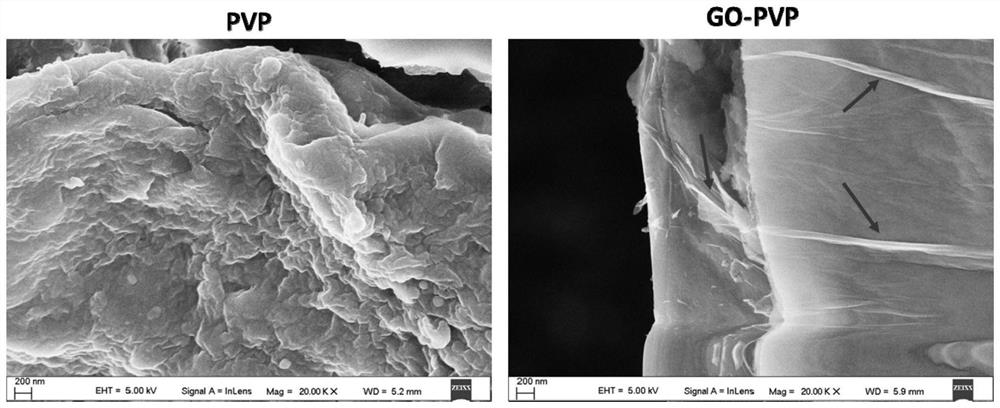
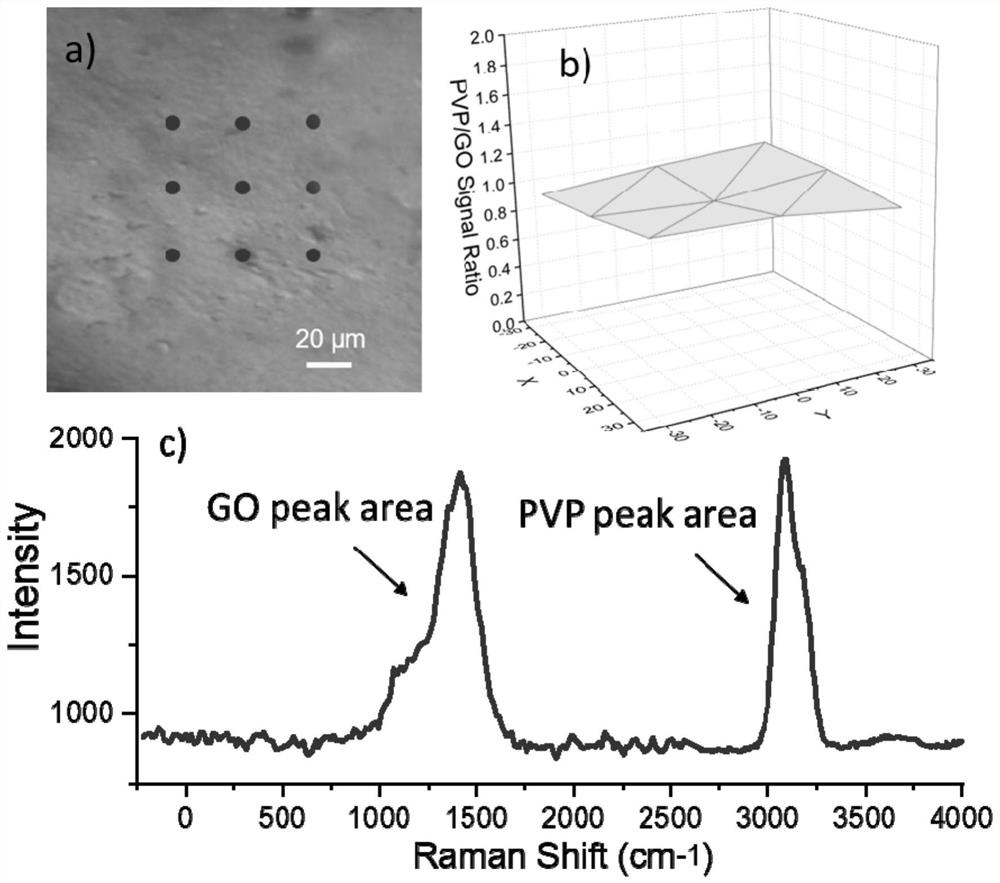
A graphene transdermal drug delivery microneedle
A kind of ene transdermal and graphene technology, which is applied in the field of medicine, can solve the problems of long time, complicated operation and high cost, and achieve good drug release ability, simple preparation method and good antibacterial performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] As a graphene transdermal delivery microneedle according to an embodiment of the present invention, the base material of the microneedle is polyvinylpyrrolidone (PVP), and graphene oxide (GO) is uniformly dispersed inside the polyvinylpyrrolidone;
[0042] Wherein, the consumption ratio of polyvinylpyrrolidone and graphene oxide is: the polyvinylpyrrolidone of 800 parts by weight and the graphene oxide of 0.01 part by weight;
[0043] The molecular weight of polyvinylpyrrolidone is 30000, namely PVP K30.
[0044] The preparation method of the graphene transdermal administration microneedle of the present embodiment comprises the following steps:
[0045] (1) Add 800 mg of polyvinylpyrrolidone (PVP K30) into 1 mL of water, and dissolve it by ultrasonication at room temperature for 15 minutes to obtain dispersion system A;
[0046] (2) Add 10 μg of graphene oxide into dispersion system A, stir and disperse evenly, remove air bubbles to obtain dispersion system B;
[004...
Embodiment 2
[0049]As a graphene transdermal delivery microneedle according to an embodiment of the present invention, the base material of the microneedle is polyvinylpyrrolidone, and graphene oxide is uniformly dispersed inside the polyvinylpyrrolidone;
[0050] Wherein, the consumption ratio of polyvinylpyrrolidone and graphene oxide is: the polyvinylpyrrolidone of 800 parts by weight and the graphene oxide of 0.1 part by weight;
[0051] The molecular weight of polyvinylpyrrolidone is 30000, namely PVP K30.
[0052] The preparation method of the graphene transdermal administration microneedle of the present embodiment comprises the following steps:
[0053] (1) Add 800 mg of polyvinylpyrrolidone into 1 mL of water, and dissolve it by ultrasonication at room temperature for 15 minutes to obtain dispersion system A;
[0054] (2) Add 100 μg of graphene oxide into dispersion system A, stir and disperse evenly, remove air bubbles to obtain dispersion system B;
[0055] (3) Add the dispers...
Embodiment 3
[0057] As a graphene transdermal delivery microneedle according to an embodiment of the present invention, the base material of the microneedle is polyvinylpyrrolidone, and graphene oxide is uniformly dispersed inside the polyvinylpyrrolidone;
[0058] Wherein, the consumption ratio of polyvinylpyrrolidone and graphene oxide is: the polyvinylpyrrolidone of 800 parts by weight and the graphene oxide of 0.5 part by weight;
[0059] The molecular weight of polyvinylpyrrolidone is 30000, namely PVP K30.
[0060] The preparation method of the graphene transdermal administration microneedle of the present embodiment comprises the following steps:
[0061] (1) Add 800 mg of polyvinylpyrrolidone into 1 mL of water, and dissolve it by ultrasonication at room temperature for 15 minutes to obtain dispersion system A;
[0062] (2) Add 500 μg of graphene oxide into dispersion system A, stir and disperse evenly, remove air bubbles to obtain dispersion system B;
[0063] (3) Add the disper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com