Ex vivo amplification culture system of umbilical cord hematopoietic stem cells and ex vivo amplification method for umbilical cord hematopoietic stem cells
A technology for hematopoietic stem cells and a culture system, which is applied in the field of umbilical cord hematopoietic stem cells in vitro expansion and culture system, can solve the problem of difficulty in meeting the large demand of HSCs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment carries out the preparation of umbilical cord blood mononuclear cells, comprising the following steps:
[0028] Add heparin sodium (20mg / L) in a 100ml glass bottle for anticoagulation, and collect the cord blood of fresh full-term pregnancy. Among them, the collection of cord blood excludes infectious diseases and various family genetic diseases, and the cord blood collection is strictly aseptic operate. The average volume of the collected cord blood is 50ml-100ml, and the cord blood is separated within 4 hours after collection. When separating, divide the umbilical cord blood into several sterile centrifuge tubes and centrifuge at 1500rpm for 10min. After centrifugation, transfer the upper layer of plasma to a new centrifuge tube, inactivate at 56°C for 30 minutes, let stand at -20°C for 10 minutes, and centrifuge at 2000rpm for 10 minutes, then take the upper layer of plasma for use. After centrifugation at 1500rpm for 10min, blood cells were obtaine...
Embodiment 2
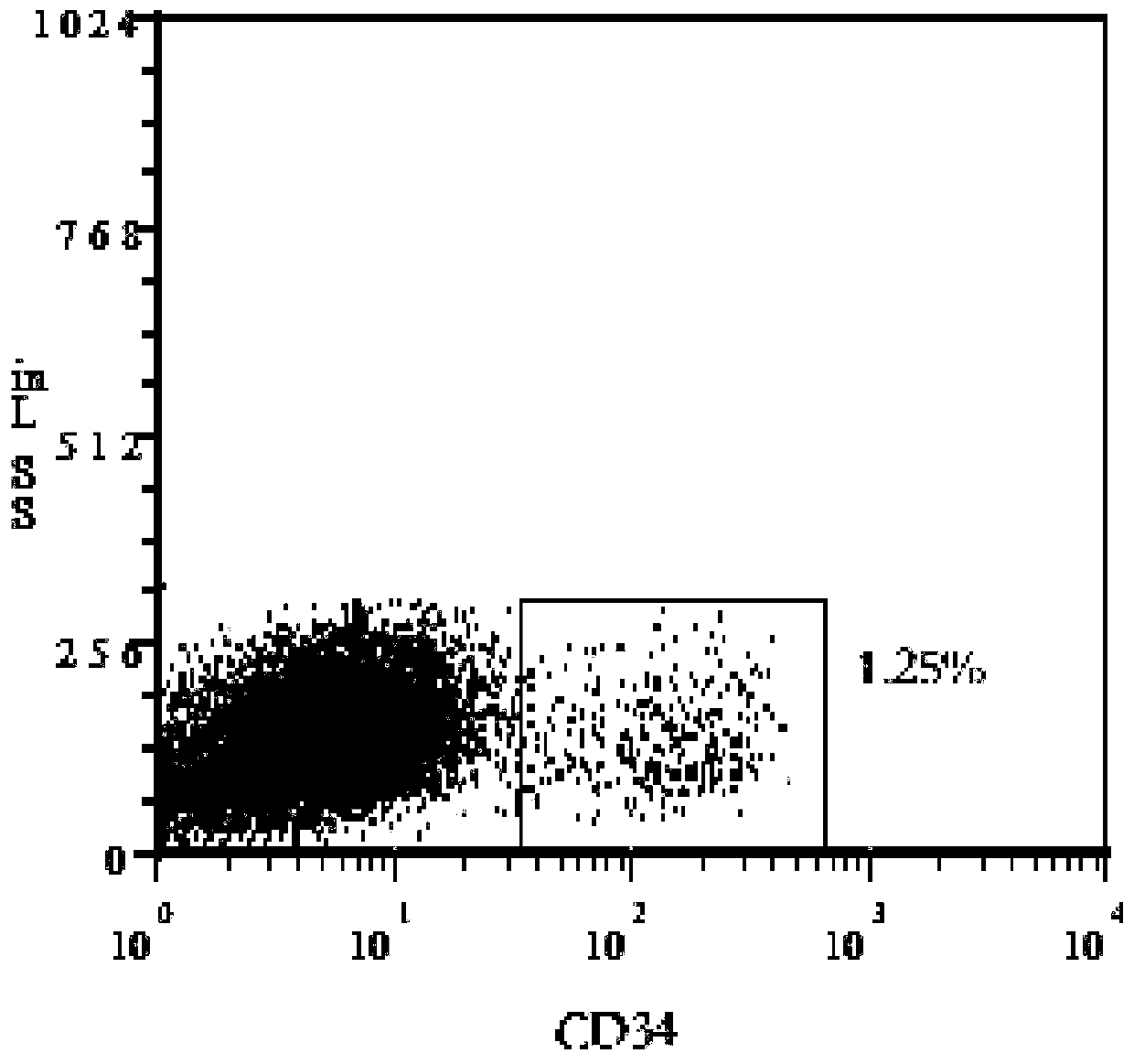
[0030] In this example, the content of CD34+ cells was detected on the umbilical cord blood mononuclear cells prepared in Example 1. The results were detected by flow cytometry figure 2 As shown, the results showed that the CD34+ cell content in cord blood mononuclear cells was 1.25%.
Embodiment 3
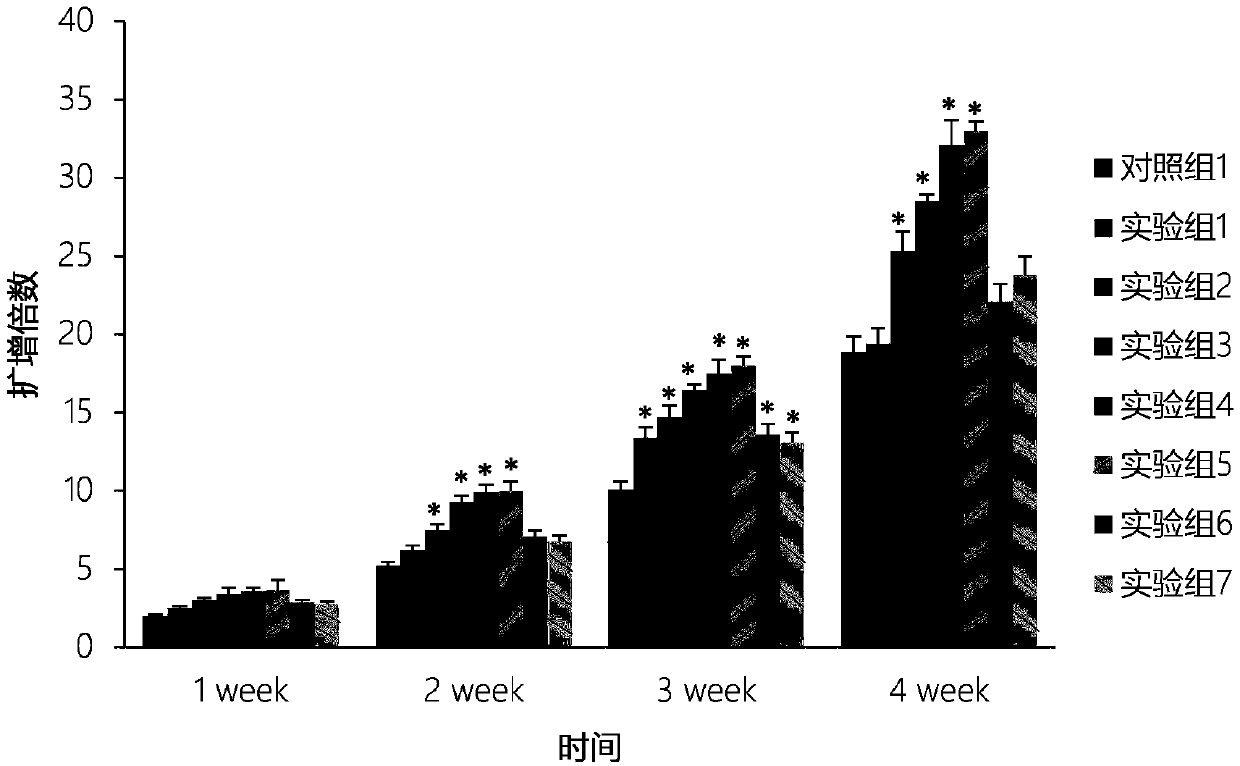
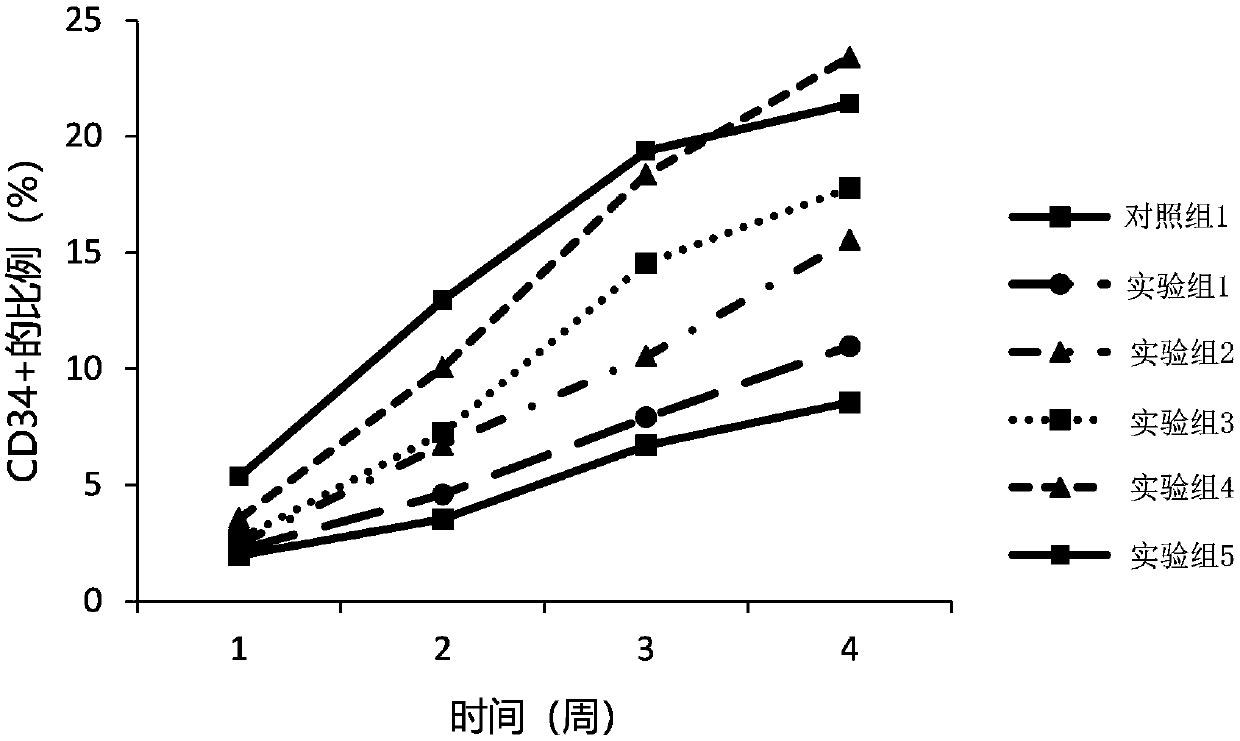
[0032] In this embodiment, the umbilical cord blood mononuclear cells prepared in Example 1 are divided into eight groups, corresponding to the following different experimental groups, cultured in different culture systems, cultured in 24-well plates, and the volume of the culture system is 0.5 ml per well, The cell seeding density was 2×10 6 cells / mL, half the volume was changed every two days. Wherein, the plasma in the culture system is the plasma prepared in Example 1. Observe the cell growth density under an inverted microscope at the 1st, 2nd, 3rd, and 4th week, and count the suspension cells at the same time. The results are as follows: figure 2 As shown, compared with control group 1, the proliferation rate of cord blood mononuclear cells in experimental group 1 to experimental group 7 was faster, indicating that G1 / S-specific cyclin-D1 (cyclins D1) and bone morphogenetic protein 4 ( Bone morphogenetic protein 4, BMP4) can promote the proliferation rate of umbilical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com