A kind of liver stem cell injection and preparation method thereof
A hepatic stem cell and cell technology, which is applied in the field of hepatic stem cell injection and its preparation, can solve the problem of not yet established a safe and reliable human hepatic stem cell line, achieve better therapeutic effect, reduce injury rate and death rate, and prolong the number of passages. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
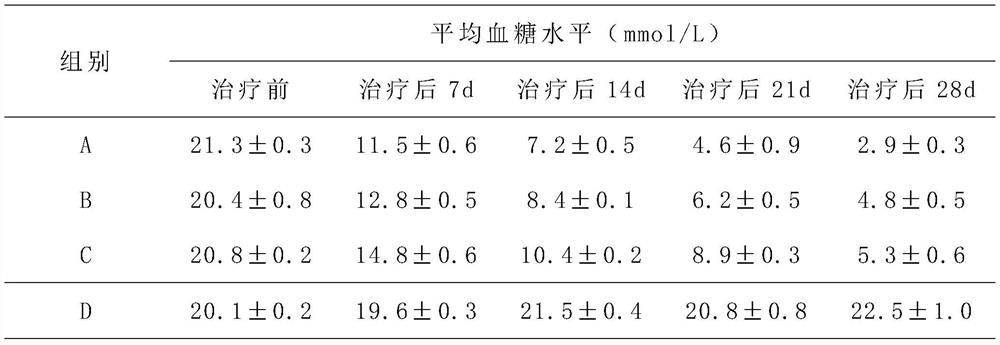
[0032] A hepatic stem cell injection for treating diabetes, each ml injection mixture contains 1×10 7 hepatic stem cells and 1×10 3 umbilical cord mesenchymal stem cells; the injection mixture consists of the following mass percentage components: 2% polyethylene glycol, 2% arginine, 3% lysine, 2% methionine, 4% % valine, 0.2% glacial acetic acid, 3% collagen, 2% glycosaminoglycan, 0.3% retinoic acid and 0.8% ethanolamine; the balance is normal saline.
Embodiment 2
[0034] A hepatic stem cell injection for treating diabetes, each ml injection mixture contains 3×10 5 hepatic stem cells and 1×10 4 umbilical cord mesenchymal stem cells; the injection mixture consists of the following mass percentage components: 8% polyethylene glycol, 5% arginine, 7% lysine, 6% methionine, 7% % valine, 0.8% glacial acetic acid, 5% collagen, 5% glycosaminoglycan, 0.3-0.5% retinoic acid and 1.2% ethanolamine; the balance is normal saline.
[0035] Preparation:
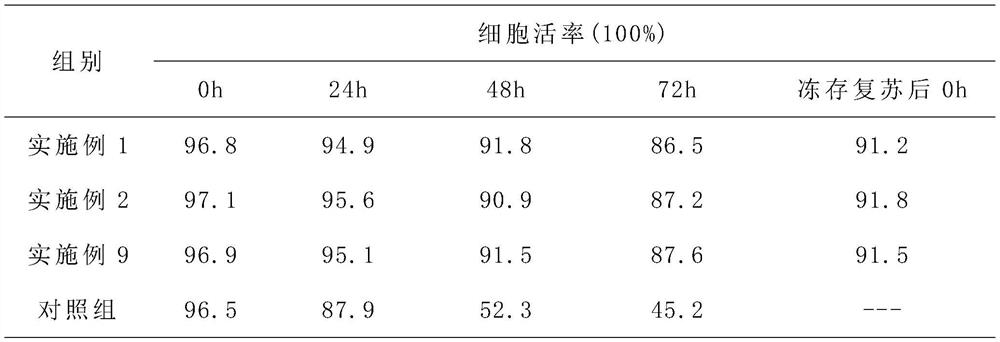
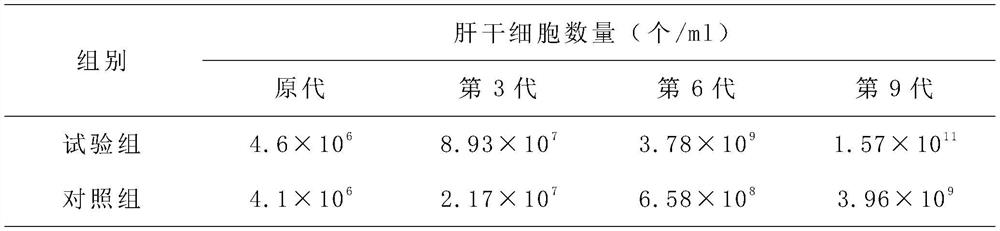
[0036] 1) Culture of liver stem cells;
[0037] 2) Culture of umbilical cord mesenchymal stem cells;
[0038] 3) Polyethylene glycol, arginine, lysine, methionine, valine, glacial acetic acid, collagen, glycosaminoglycan, retinoic acid and ethanolamine are dissolved in normal saline to prepare a compound solution, Pre-cool at 4°C for later use;
[0039] 4) Resuspend the hepatic stem cells obtained in step 1) and the umbilical cord mesenchymal stem cells obtained in step 2) in the compound solution...
Embodiment 3
[0041] A hepatic stem cell medium, based on DMEM / F12 medium, comprising the following components at concentrations: 6mg / ml hyaluronic acid, 60ng / ml hepatocyte growth factor, 50ng / ml basic fibroblast growth factor, 10μg / ml ml recombinant human bone morphogenetic protein, 300 μg / ml 2′,3′-dideoxyadenosine-5-triphosphate, 7 mg / ml ferric citrate, 10 mg / ml carboxymethylcellulose sodium and 3 mg / ml L-carnitine .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com