Nitrogen-doped porous carbon non-metal catalyst and preparation method thereof, and application of nitrogen-doped porous carbon non-metal catalyst in oxidation-reduction reaction
A non-metal catalyst, nitrogen-doped porous carbon technology, applied in electrical components, battery electrodes, circuits, etc., to achieve high-scale production value, abundant and controllable raw materials, and optimized catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Pour 80mL of 0.5mol / L glucose solution into the liner of a 100mL polytetrafluoroethylene reactor, seal it in a stainless steel reactor and put it in an oven at 180°C for 10 hours; after the reaction, the obtained materials are centrifuged at 9500rpm and washed with deionized water for 3 Second, dry at 60°C for 24 hours in a blast oven to obtain hydrothermal glucose carbon spheres.
[0031] Grind and mix the prepared hydrothermal glucose carbon spheres with urea and zinc chloride at a mass ratio of 1:10:1, put them into an alumina ark, and then put them into a quartz tube in a tube furnace. Nitrogen was passed for more than 60 minutes to ensure that the air was exhausted, and the temperature in the tube furnace was raised to 900°C at a heating rate of 5°C / min. After holding for 2 hours, it was cooled with the furnace to obtain an electrocatalyst for oxygen reduction reaction-nitrogen-doped porous carbon. catalyst.
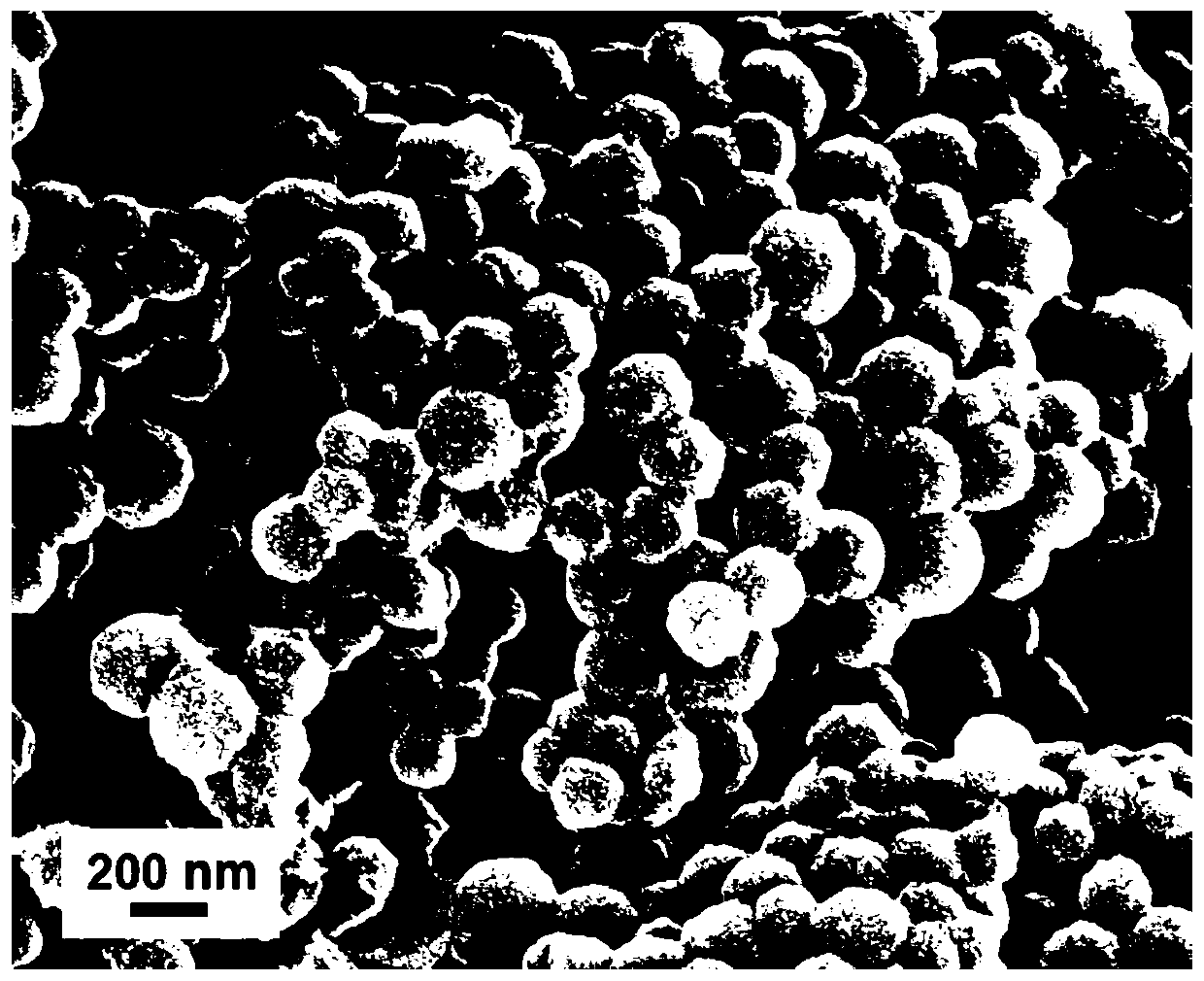
[0032] figure 1 It is a scanning electron microscope ...
Embodiment 2
[0036] Pour 80mL of 0.5mol / L glucose solution into a 100mL polytetrafluoroethylene reactor liner, seal it in a stainless steel reactor and put it in an oven at 180°C for 10h; Wash 3 times and dry at 60°C for 24 hours in a blast oven to obtain hydrothermal glucose carbon spheres.
[0037] Grind and mix the prepared hydrothermal glucose carbon spheres with urea and zinc chloride at a mass ratio of 1:10:1, put them into an alumina ark, and then put them into a quartz tube in a tube furnace. Nitrogen was passed for more than 60 minutes to ensure that the air was exhausted, and the temperature in the tube furnace was raised to 1000°C at a heating rate of 5°C / min. After holding for 2 hours, it was cooled with the furnace to obtain an electrocatalyst for oxygen reduction reaction-nitrogen-doped porous carbon. catalyst.
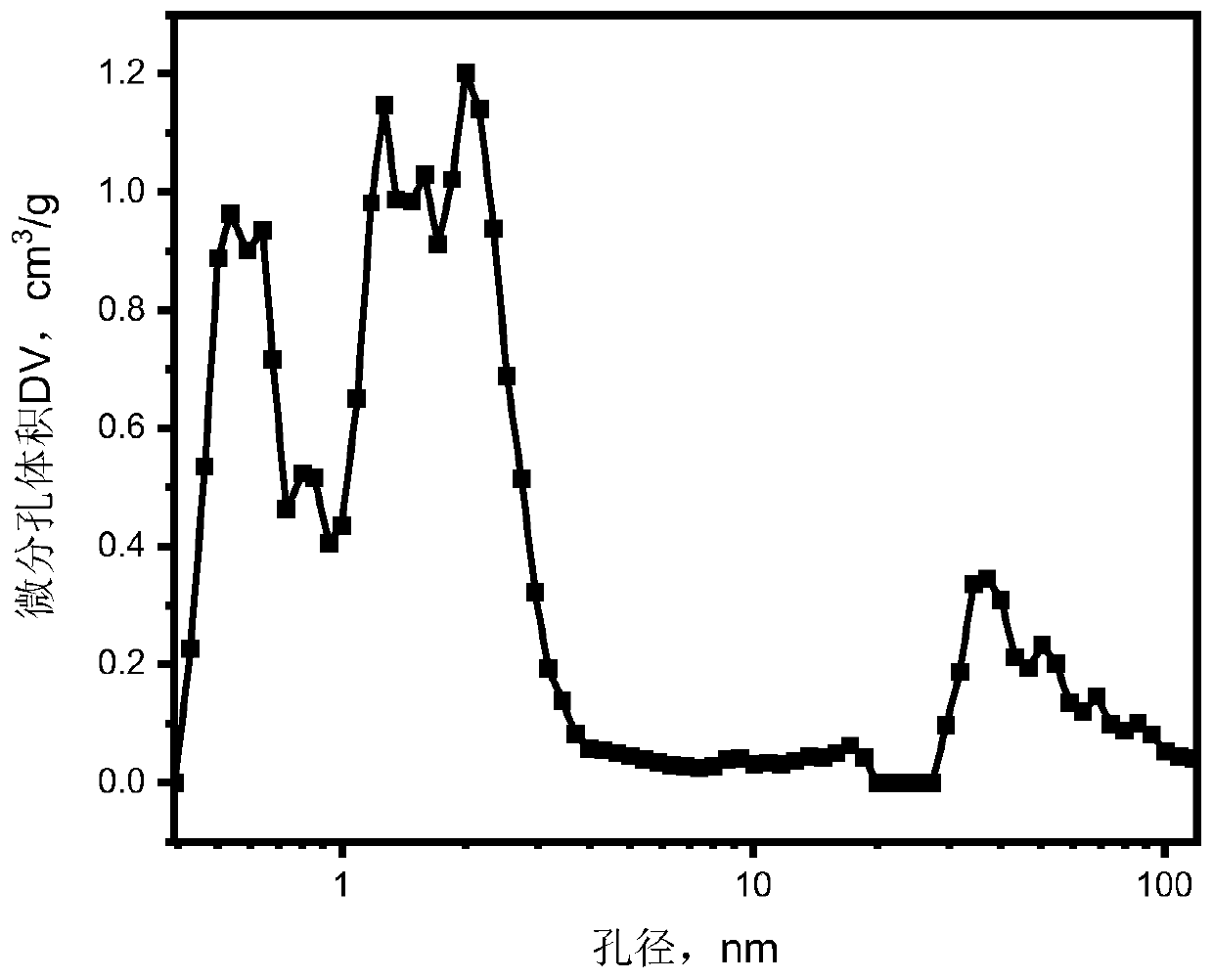
[0038] Figure 4 It is the nitrogen adsorption-desorption curve of the oxygen reduction reaction electrocatalyst nitrogen-doped porous carbon obtained in Example 2. ...
Embodiment 3
[0040] Pour 80mL of 0.5mol / L glucose solution into a 100mL polytetrafluoroethylene reactor liner, seal it in a stainless steel reactor and put it in an oven at 180°C for 10 hours; after the reaction, the obtained materials are centrifuged at 9500rpm and washed with deionized water in sequence 3 times, drying in a blast oven at 60°C for 24 hours to obtain hydrothermal glucose carbon spheres.
[0041] Grind and mix the prepared hydrothermal glucose carbon spheres with urea and zinc chloride at a mass ratio of 1:10:1, put them into an alumina ark, and then put them into a quartz tube in a tube furnace. Nitrogen was passed for more than 60 minutes to ensure that the air was exhausted, and the temperature in the tube furnace was raised to 900°C at a heating rate of 5°C / min. After holding for 2 hours, it was cooled with the furnace to obtain an electrocatalyst for oxygen reduction reaction and a nitrogen-doped porous carbon catalyst. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap