Stabilized antibody compositions and methods of producing same
A stable, gas-based technology, applied in chemical instruments and methods, devices that make medicines into special physical or ingestible forms, specific peptides, etc., can solve the problems of headspace gas oxygen concentration, percentage increase, not provided, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] Preparation of antigen binding domains and construction of bispecific molecules
[0100] The antigen-binding domains specific for a particular antigen can be prepared by any antibody production technique known in the art. Once obtained, conventional methods can be used to appropriately arrange two different antigen-binding domains specific for two different antigens relative to each other to produce the bispecific antigen-binding molecule of the present invention. In certain embodiments, one or more individual components (eg, heavy and light chains) of the multispecific antigen binding molecules of the invention are derived from chimeric antibodies, humanized antibodies, or fully human antibodies. Methods of preparing such antibodies are well known in the art. For example, you can use VELOCIMMUNE TM The technique prepares one or more heavy chains and / or light chains of the bispecific antigen binding molecules of the present invention. Use Velocimmune TM Technology (or ...
Embodiment 1
[0113] Example 1: Reducing oxygen in the headspace of medicines
[0114] In this embodiment, a standard GMP freeze-drying chamber is used as a vacuum chamber, which is equipped with a Pirani vacuum gauge for measurement and a needle valve for pressure control. During the two cycles, the bispecific antibody with a concentration of 2 mg / ml in the liquid formulation was packaged in a vial with a rubber stopper on the exhaust plunger and covered with nitrogen to reduce the oxygen in the headspace from -21% Reduce to about 0.25%.
[0115] Table 1: Non-oxidizing gas covering treatment
[0116]
[0117] Once the vial is placed in the chamber, a vacuum is applied (step 6) to remove the gas from the chamber (it starts with -21% oxygen in air). The pressure (100,000 microbars) is higher than the vapor pressure of water to avoid evaporation, foam formation and potential splashing, and is measured with a Pirani vacuum gauge. Under ordinary freeze-drying conditions, there is a vacuum that reac...
Embodiment 2
[0138] Example 2: Stability test of medicine
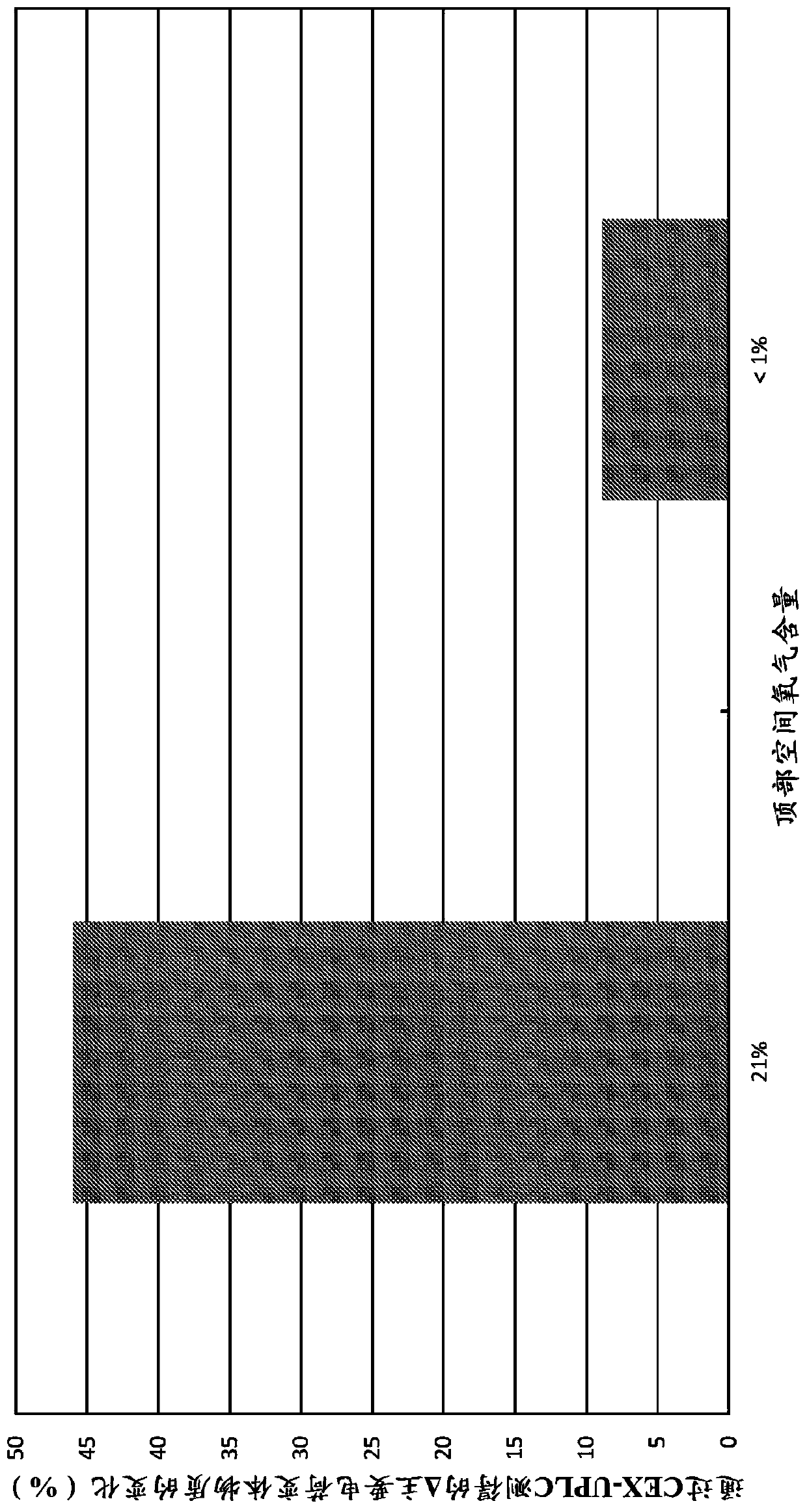
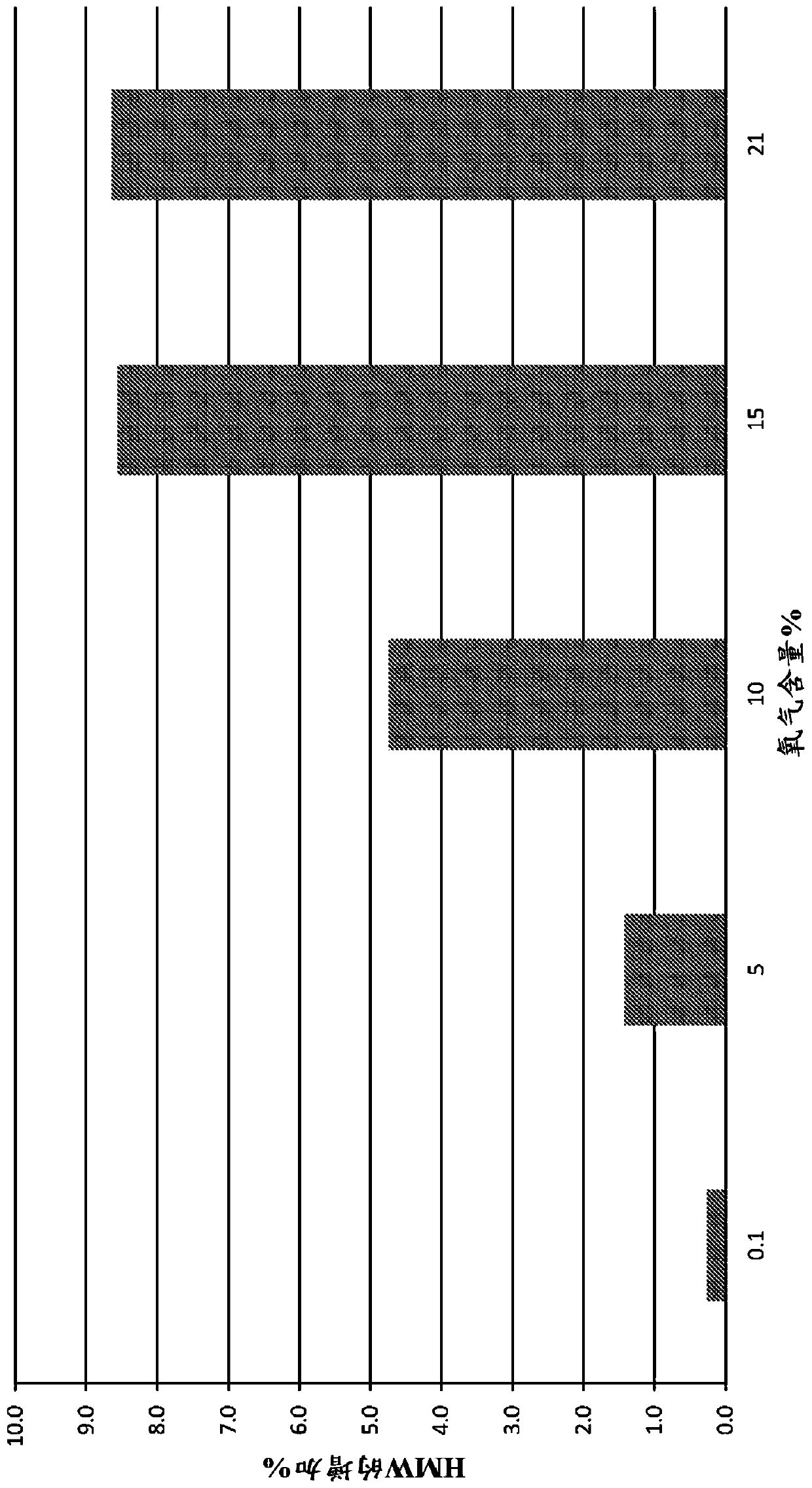
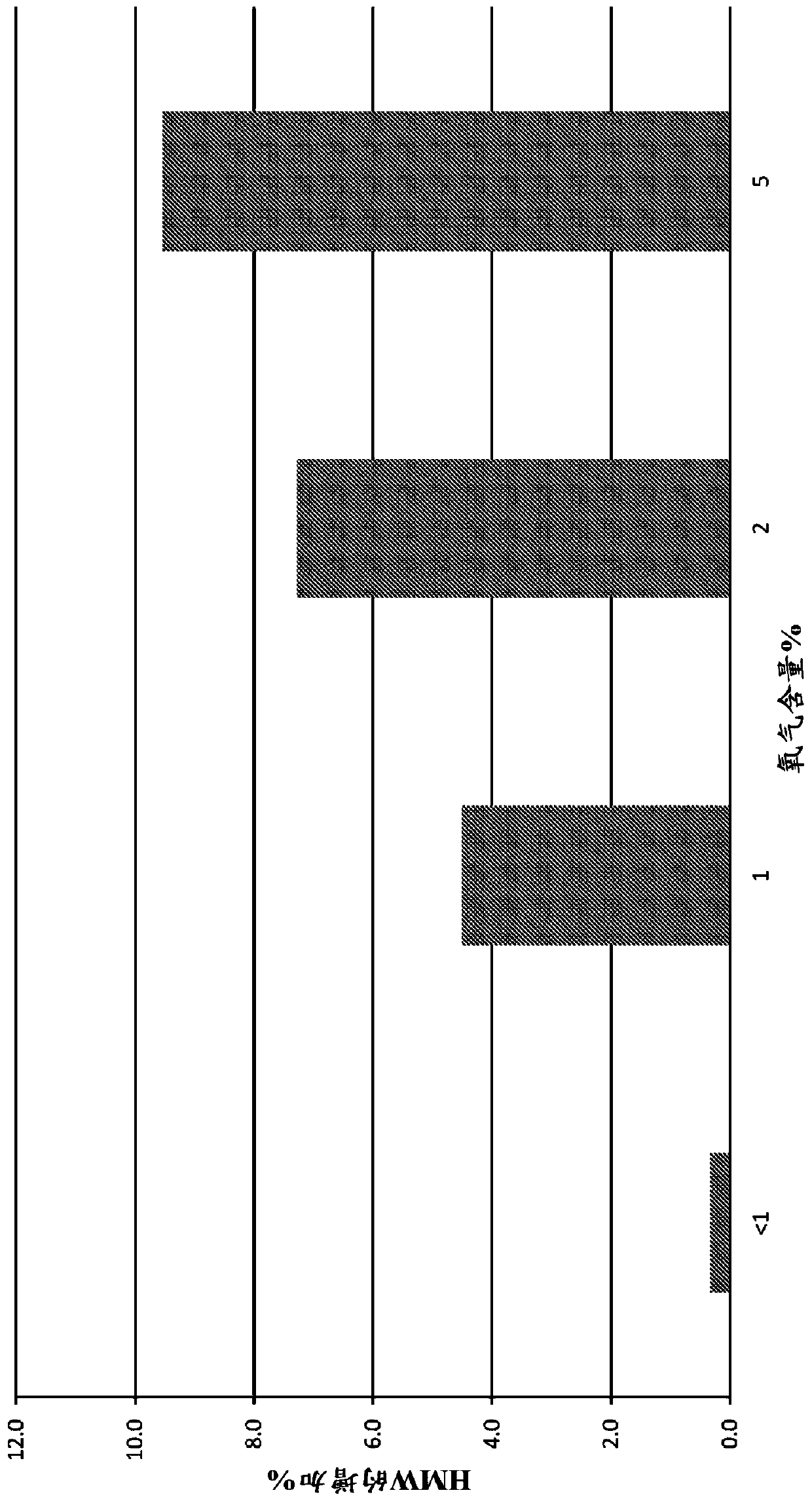
[0139] Using different concentrations of headspace oxygen, the stability analysis of various drugs prepared using the method discussed in Example 1 was carried out and compared with the control where the headspace oxygen was at or close to the atmospheric level (~21%) For comparison. In some cases (noted here), argon is used instead of nitrogen in the charging part of the method. Size exclusion ultra performance liquid chromatography (SE-UPLC) was used to detect high molecular weight (HMW) substances, and cation exchange ultra performance liquid chromatography (CEX-UPLC) was used to detect charge variant substances.
[0140] Such as figure 1 As shown, reducing the headspace oxygen content from 21% to less than 1% by nitrogen blanketing reduced the degradation of bispecific antibodies observed by CEX-UPLC after 31 months storage at 5°C. In the case of an oxygen content of 21%, the percentage change of the main charge variant substanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com