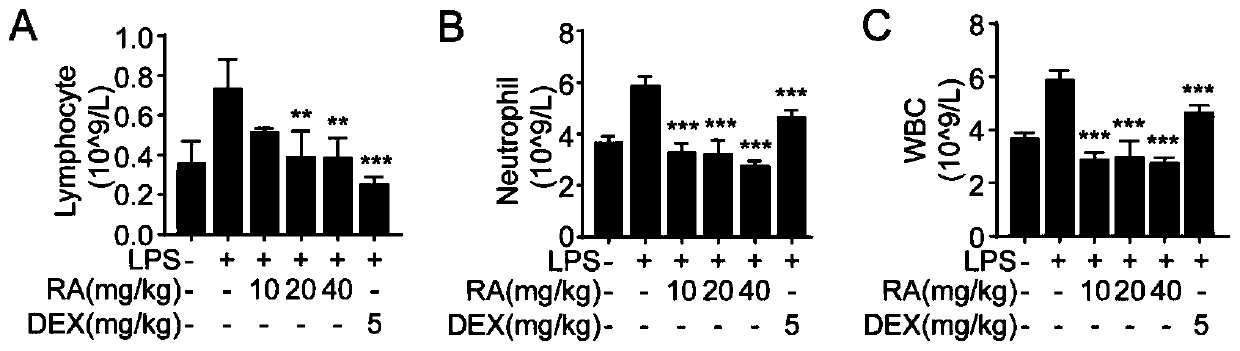
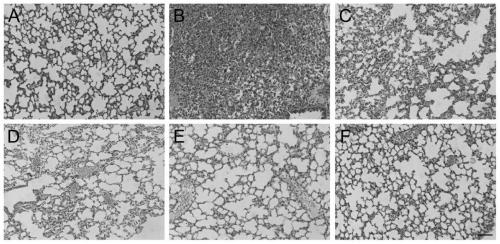
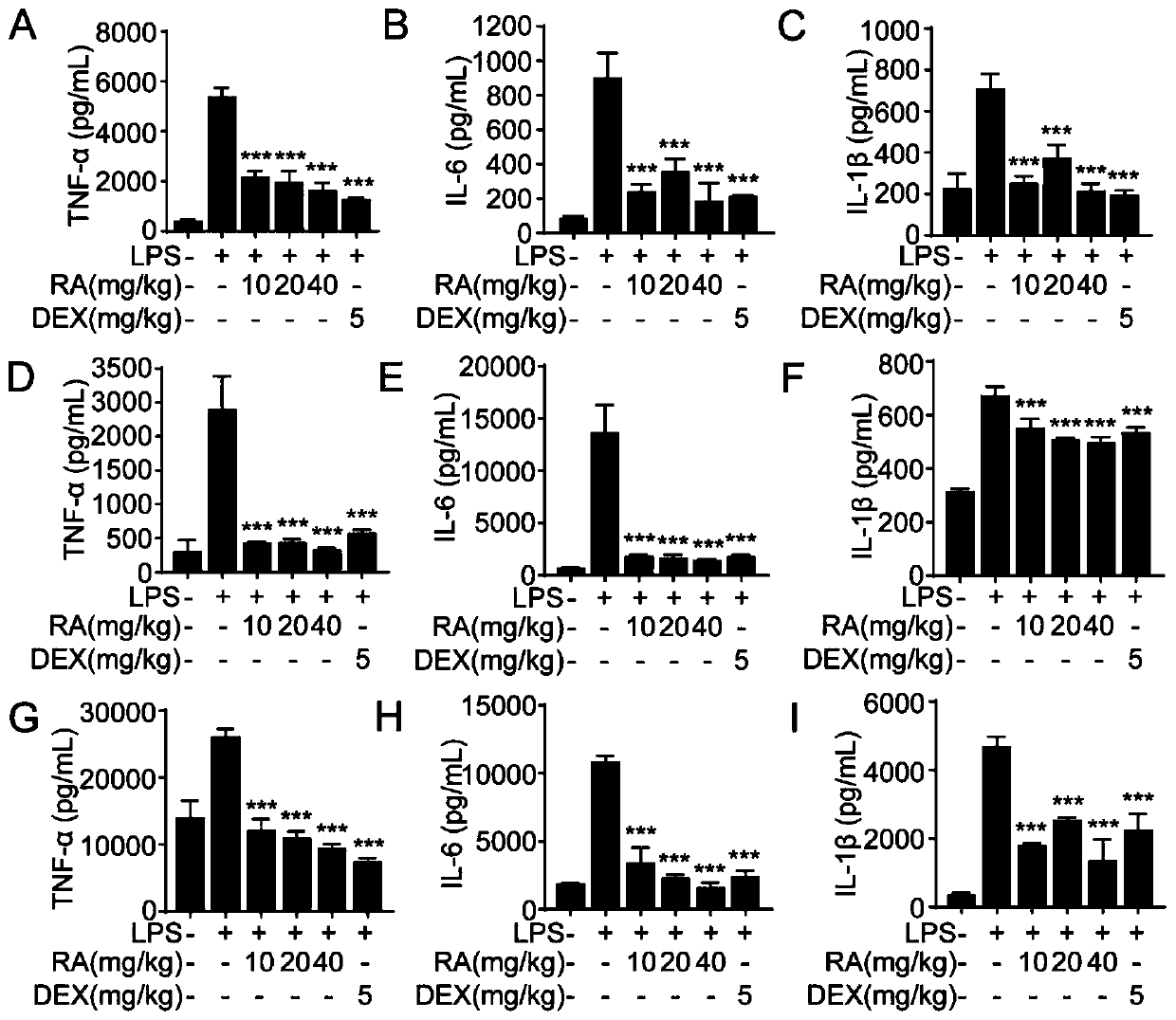
Purpose of rotundic acid to preparation of medicines for treating pneumonia
A technology for the use of iron hollylic acid, which is applied in the field of preparing drugs for the treatment of pneumonia, can solve the problems such as the treatment of bacterial pneumonia that has not been reported, and achieve the effect of improving the survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be further described in detail below in conjunction with the accompanying drawings, so that those skilled in the art can implement it with reference to the description.
[0025] It should be understood that terms such as "having", "comprising" and "including" as used herein do not entail the presence or addition of one or more other elements or combinations thereof.
[0026] It should be noted that the experimental methods described in the following embodiments, unless otherwise specified, are conventional methods, and the reagents and materials, unless otherwise specified, can be obtained from commercial sources.
[0027] 1 material
[0028] 1.1 Experimental animals
[0029] SPF grade healthy BALB / C mice, weighing 20-22g, 7-8 weeks old, male, were purchased from Hunan Slack Jingda Experimental Animal Co., Ltd. All experimental animals were raised in a controlled environment, room temperature 18-24°C, The humidity was 40%-50%. During the exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com