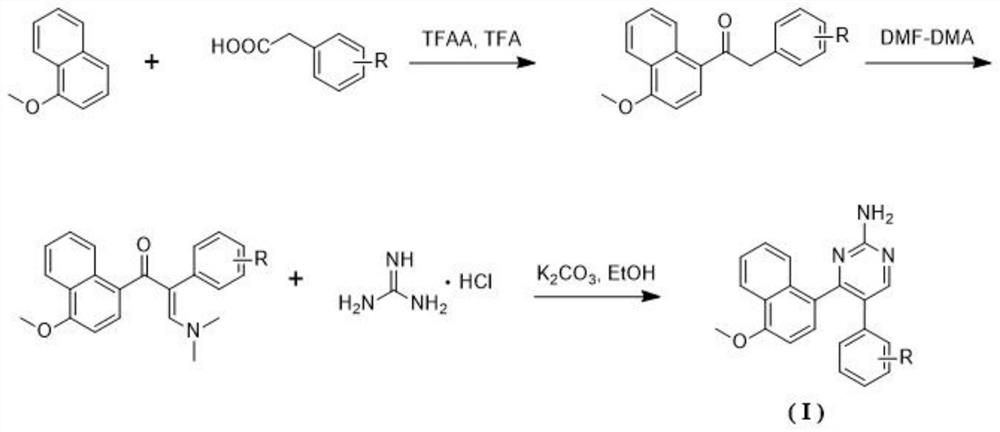
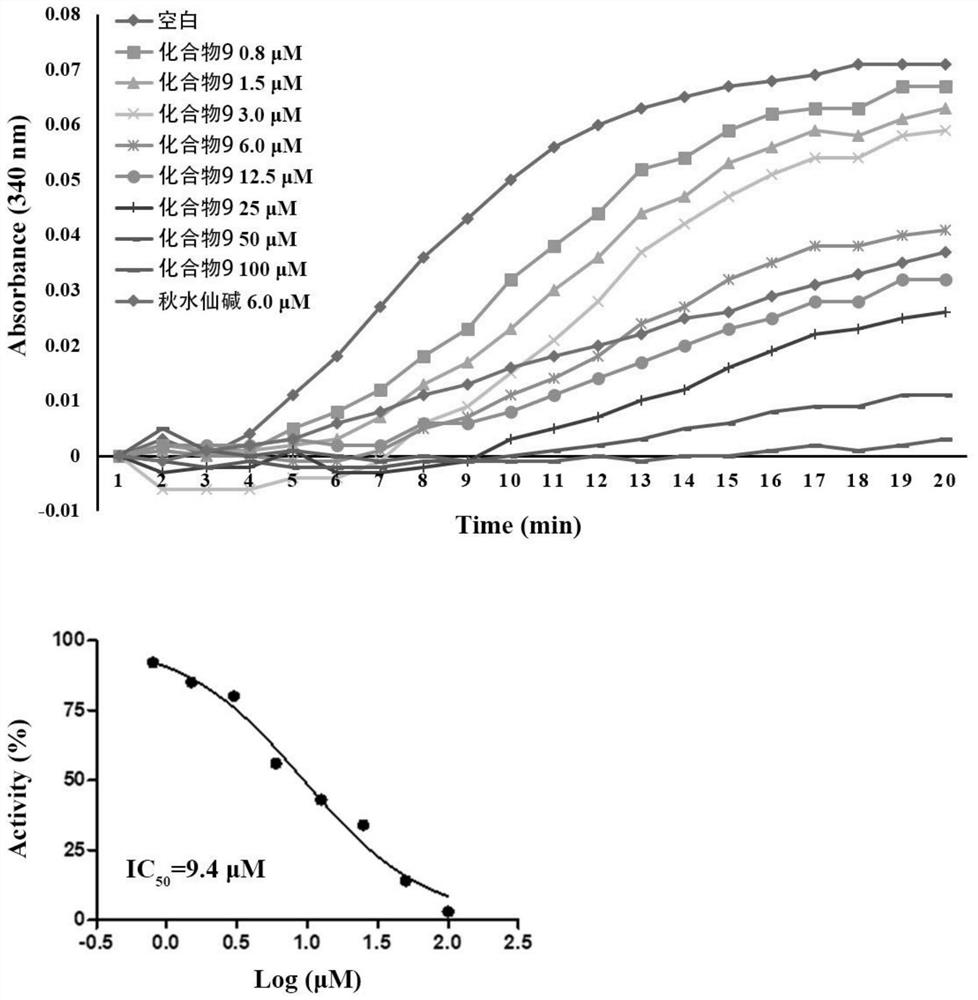
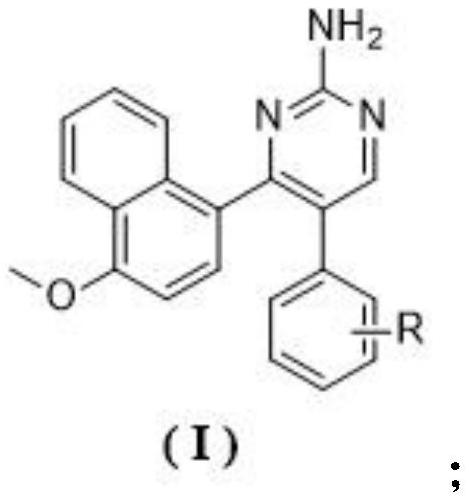
A kind of naphthalene ring-aminopyrimidine type compound and its preparation method and application
A technology for aminopyrimidines and compounds, applied in the field of compound preparation, can solve problems such as difficult sources, high toxicity, and drug resistance, and achieve the effects of simple preparation methods, short synthetic routes, and low requirements for experimental equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of a kind of 4-(4-methoxynaphthalen-1-yl)-5-(3,4,5-trimethoxyphenyl)pyrimidin-2-amine (1)
[0038] The structural formula of compound 1 is as follows:
[0039]
[0040] Concrete preparation steps are as follows:
[0041] Step 1: Add 5ml of trifluoroacetic acid to 1-methoxynaphthalene (1.5mmol), 3,4,5-trimethoxyphenylacetic acid (1.0mmol), and trifluoroacetic anhydride (2.0mmol), and stir at room temperature for 12 hours, spin-dried, separated and purified by silica gel column chromatography to obtain 1-(4-methoxynaphthalen-1-yl)-2-(3,4,5-trimethoxyphenyl)ethan-1-one.
[0042] Step 2: Mix 1-(4-methoxynaphthalen-1-yl)-2-(3,4,5-trimethoxyphenyl)ethan-1-one (1.0 mmol), DMF-DMA (10.0 mmol) was placed in a reaction flask, refluxed for 24 hours, and was spin-dried to obtain (E)-3-(dimethylamino)-1-(4-methoxynaphthalene-1-yl)-2-(3 ,4,5-trimethoxyphenyl)prop-2-en-1-one.
[0043] Step 3: Add (E)-3-(dimethylamino)-1-(4-methoxynaphthalen-1-yl)-2-(3,4,5-trimethoxy...
Embodiment 2
[0049] Preparation of a kind of 4-(4-methoxynaphthalen-1-yl)-5-(4-methoxyphenyl)pyrimidin-2-amine (2)
[0050] The structural formula of compound 2 is as follows:
[0051]
[0052] Concrete preparation steps are as follows:
[0053] Step 1: Add 5ml of trifluoroacetic acid to 1-methoxynaphthalene (1.5mmol), 4-methoxyphenylacetic acid (1.0mmol), and trifluoroacetic anhydride (2.0mmol), stir at room temperature for 12 hours, spin dry , separated and purified by silica gel column chromatography to obtain 1-(4-methoxynaphthalen-1-yl)-2-(4-methoxyphenyl)ethan-1-one.
[0054] Step 2: Place 1-(4-methoxynaphthalen-1-yl)-2-(4-methoxyphenyl)ethan-1-one (1.0mmol), DMF-DMA (10.0mmol) in In the reaction bottle, reflux reaction for 24 hours, and spin dry to obtain (E)-3-(dimethylamino)-1-(4-methoxynaphthalene-1-yl)-2-(4-methoxy phenyl)prop-2-en-1-one.
[0055] Step 3: Add (E)-3-(dimethylamino)-1-(4-methoxynaphthalen-1-yl)-2-(4-methoxyphenyl)prop-2-ene-1 - Put ketone (0.5mmol), guanid...
Embodiment 3
[0060] Preparation of a kind of 4-(4-methoxynaphthalene-1-yl)-5-(4-methylphenyl)pyrimidin-2-amine (3)
[0061] The structural formula of compound 3 is as follows:
[0062]
[0063] Concrete preparation steps are as follows:
[0064] Step 1: Add 5ml of trifluoroacetic acid to 1-methoxynaphthalene (1.5mmol), 4-methylphenylacetic acid (1.0mmol), and trifluoroacetic anhydride (2.0mmol), stir at room temperature for 12 hours, spin dry, Separation and purification by silica gel column chromatography gave 1-(4-methoxynaphthalen-1-yl)-2-(4-methylphenyl)ethan-1-one.
[0065] Step 2: 1-(4-methoxynaphthalen-1-yl)-2-(4-methylphenyl)ethan-1-one (1.0 mmol), DMF-DMA (10.0 mmol) were placed in the reaction In the bottle, reflux for 24 hours, and spin dry to obtain (E)-3-(dimethylamino)-1-(4-methoxynaphthalene-1-yl)-2-(4-methylphenyl ) prop-2-en-1-one.
[0066] Step 3: Add (E)-3-(dimethylamino)-1-(4-methoxynaphthalen-1-yl)-2-(4-methylphenyl)prop-2-ene-1- Ketone (0.5mmol), guanidine hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com