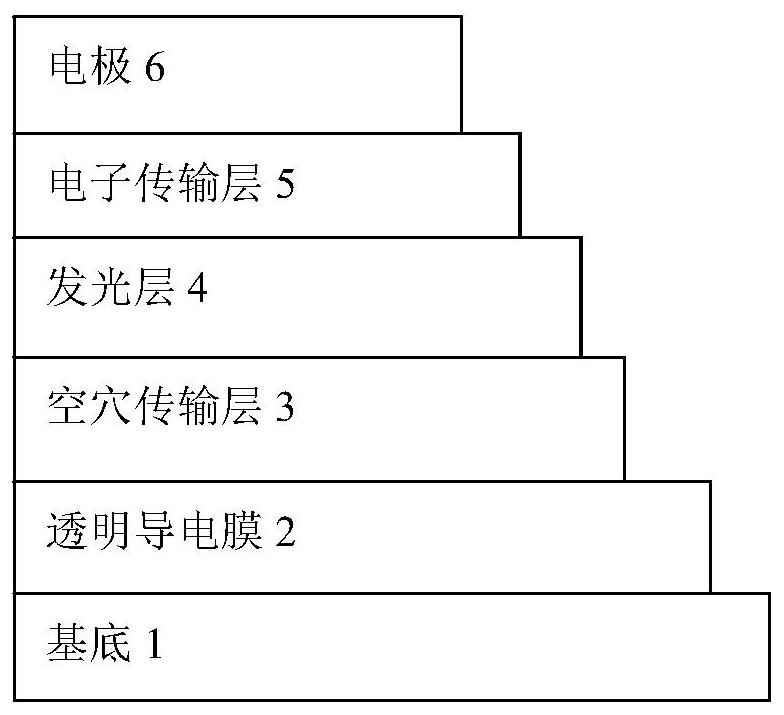
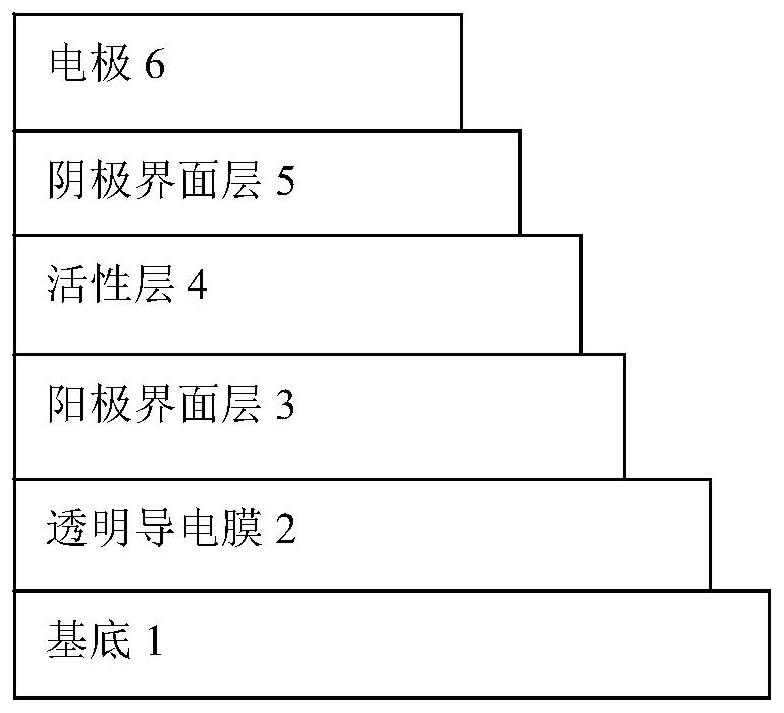
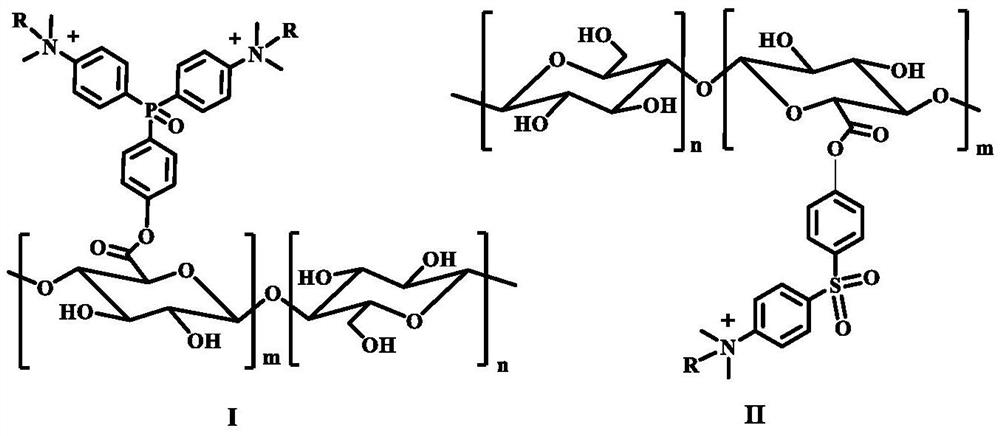
A kind of cellulose electron transport polymer and its preparation method and application
An electron transport, polymer technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of cellulose and its derivatives that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1 Synthesis of the compound of formula I-1
[0092] In this embodiment, the structural formula of the compound of formula I-1 is:
[0093]
[0094] The preparation method of the above-mentioned compound of formula I-1 comprises the following steps:
[0095] Step 1: Synthesis of intermediate formula, bis[4-(dimethylamino)phenyl]-4-hydroxyphenylphosphine
[0096] 1 serving of starting material Dissolve 2 parts of p-iodophenyldimethylamine in 250 mL of tetrahydrofuran solution, under argon protection, cool down to -75°C, stir at this temperature for 60 minutes, and add 4 parts of tert-butyllithium n-hexane solution dropwise In the reaction system, the reaction system was then warmed to room temperature and stirred for 60 minutes;
[0097] Then the reaction system was placed at -75 ° C and stirred for 30 minutes, 1 part of phosphorus trichloride n-pentane solution was added dropwise to the reaction system, and after warming up to room temperature, the react...
Embodiment 2
[0107] Example 2 Synthesis of compound of formula I-2
[0108] In the present embodiment, the structural formula of the compound of formula I-2 is:
[0109]
[0110] Step 1: Synthesis of intermediate bis[4-(dimethylamino)phenyl]-4-hydroxyphenylphosphine
[0111] Combine 2 servings of starting material 4 parts of p-iodophenyldimethylamine were dissolved in 250mL of tetrahydrofuran solution, protected by argon, cooled to -75°C, stirred at this temperature for 120 minutes, 8 parts of tert-butyllithium n-hexane solution was added dropwise In the reaction system, the reaction system was subsequently warmed to room temperature and stirred for 1200 minutes;
[0112] Then the reaction system was placed at -75 ° C and stirred for 30 minutes, 2 parts of phosphorus trichloride n-pentane solution was added dropwise to the reaction system, and after warming up to room temperature, the reaction was performed for 18 hours;
[0113] Then add 6 parts of hydrogen peroxide to the above ...
Embodiment 3
[0122] Synthesis of embodiment 3 formula Ⅱ-1 compound
[0123] In the present embodiment, the structural formula of the compound of formula II-1 is:
[0124]
[0125] The preparation method of above-mentioned formula II-1 compound comprises the steps:
[0126] Step 1: Synthesis of intermediate 4-((4-dimethylamino)phenyl)sulfone)phenol
[0127] 1 part of starter Dissolve 1 part of p-iodophenyldimethylamine and 0.1 part of cesium carbonate in 250 mL of tetrahydrofuran solution, under argon protection, stir and heat up to reflux, and react for 12 hours; then add 2 parts of hydrogen peroxide to the above solution, and stir at room temperature After 60 minutes, the reaction was stopped, and after the solvent was removed, the intermediate 4-((4-dimethylamino)phenyl)sulfone)phenol was obtained as a white powder through column separation and purification, with a yield of 75%.
[0128] Mass spectrum [M] + : 275.08; 1 H NMR (CDCl 3 ): δ (ppm) = 7.82 (d, J = 8.82Hz, 2H), 7.32...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com