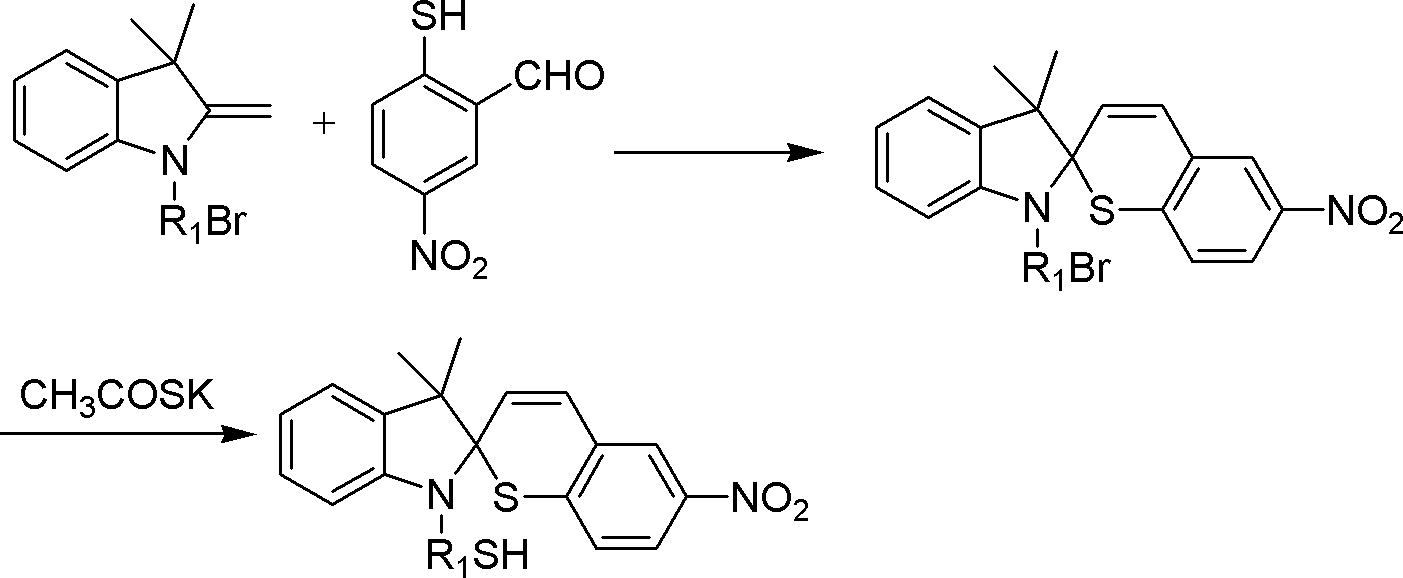
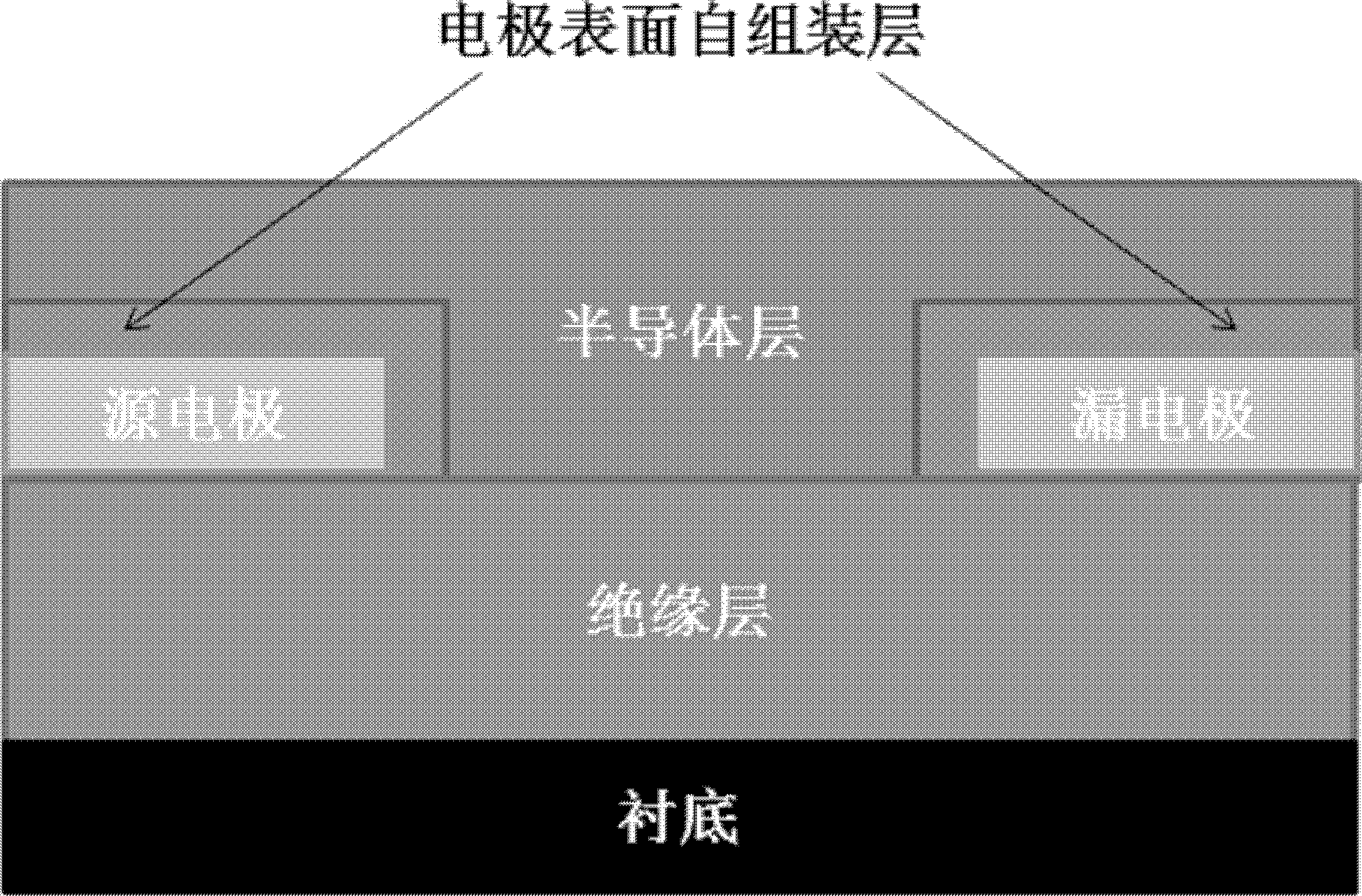
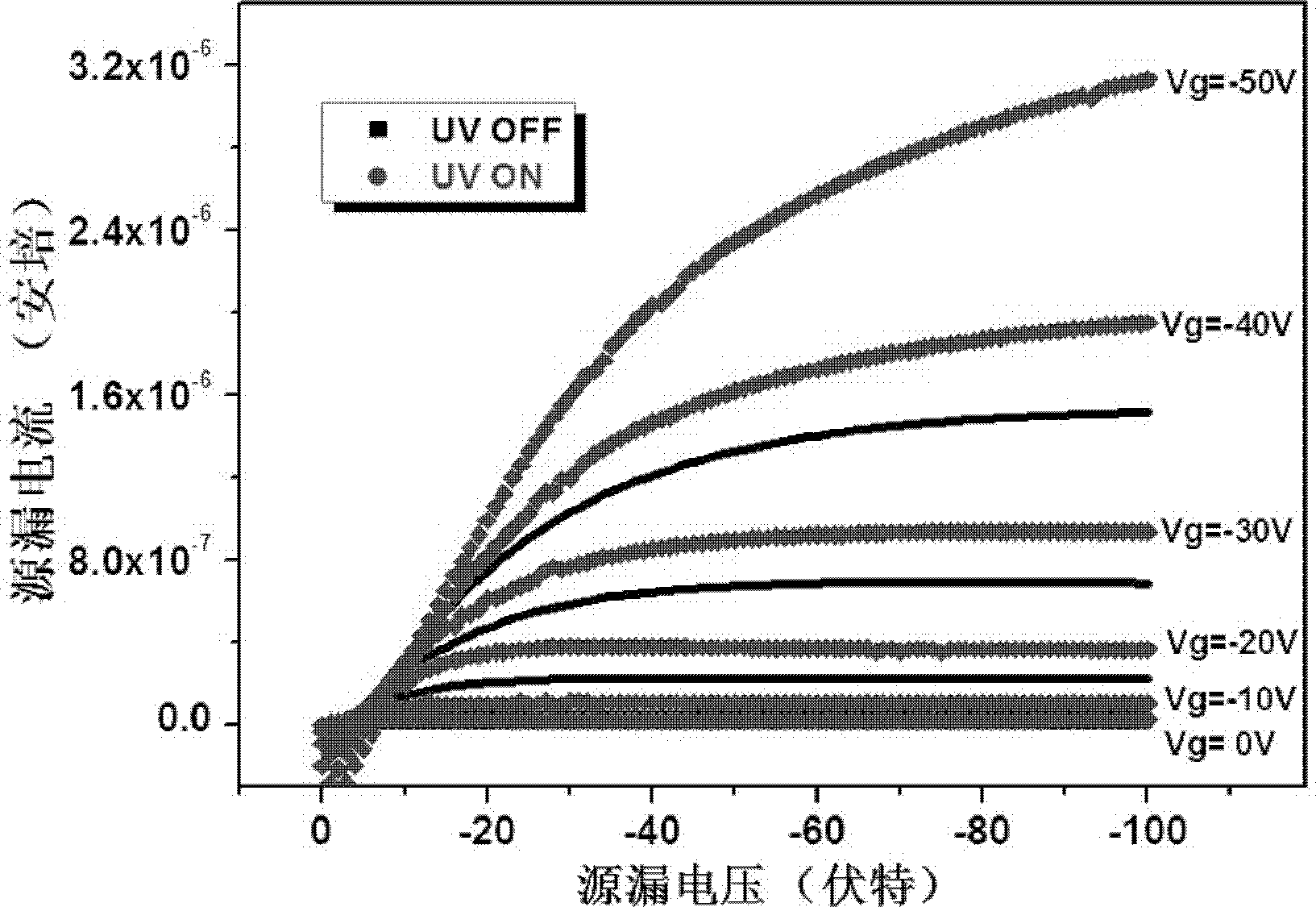
Thiol spirothiopyran compound and preparation method and application thereof
A compound and mercapto spiro technology, which are applied in the fields of mercapto spiro thiopyran compounds and their preparation and application, and achieve the effects of good binding ability, low cost and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1, Synthesizing the compound shown in formula a and preparing p-type photoelectric function organic field effect transistor
[0039]
[0040] formula a
[0041] (1) compound shown in synthetic formula a:
[0042] Under the protective condition of nitrogen, bromododecyl N-substituted indole (R 1 =-C 12 h 24 ) (4.052g, 10mmol) and o-aldehyde p-nitrothiophenol (2.198g, 12mmol) were dissolved in ethanol (30mL), heated to 60°C, stirred for 5 hours; the system was cooled to room temperature, filtered, and the obtained Solid is the compound shown in formula III (R 1 =-C 12 h 24 ), dissolve the compound shown in formula III in ethanol, add potassium thioacetate (3.426g, 30mmol), heat up to 60°C, stir for 0.5 hour, cool to room temperature, filter, add potassium hydroxide in the filtrate, stir, Spin to dry the ethanol solvent, dissolve the solid in dichloromethane, add dilute hydrochloric acid with pH = 4.0 to the solution, separate and extract, combine the org...
Embodiment 2
[0054] Example 2, Synthesizing the compound shown in formula b and preparing p-type photoelectric function organic field effect transistor
[0055]
[0056] formula b
[0057] (1) compound shown in synthetic formula b:
[0058] Under the protective condition of nitrogen, bromododecyl N substituted indole (R 1 =-C 16 h 32 ) (4.613g, 10mmol) and o-aldehyde p-nitrothiophenol (2.198g, 12mmol) were dissolved in ethanol (30mL), heated to 60°C, and stirred for 5 hours; the system was cooled to room temperature, filtered, and the obtained Solid is the compound shown in formula III (R 1 =-C 16 h 32 ), dissolve the compound shown in formula III in ethanol, add potassium thioacetate (3.426g, 30mmol), heat up to 60°C, stir for 0.5 hour, cool to room temperature, filter, add potassium hydroxide in the filtrate, stir, Spin to dry the ethanol solvent, dissolve the solid in dichloromethane, add dilute hydrochloric acid with pH = 4.0 to the solution, separate and extract, combine the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com