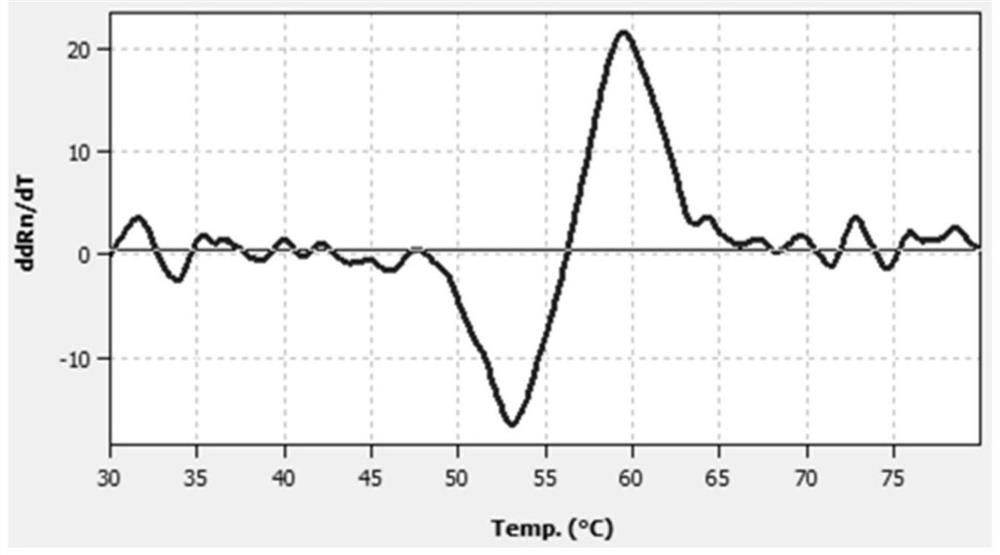
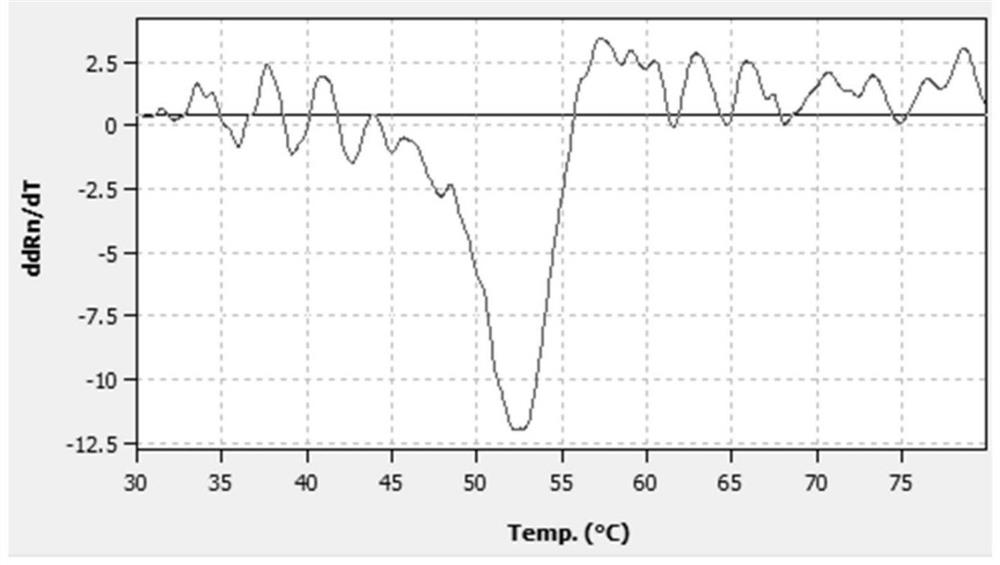
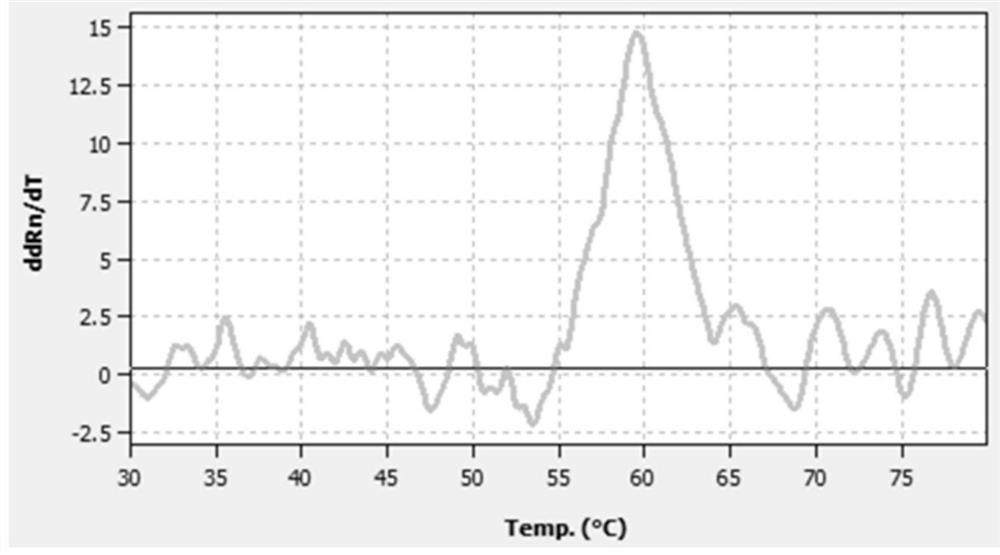
A probe composition for detecting drug-induced deafness gene and its application
A technology for drug-induced deafness and detection probe, applied in the biological field, can solve the problems of pollution, unintuitive results, complicated operation, etc., and achieve the effects of avoiding cross-contamination, intuitive interpretation of results, and strong anti-interference ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The composition of embodiment 1 kit
[0086] This embodiment provides a kit for detecting the C1494T and A1555G mutation sites of the mitochondrial DNA 12S rRNA of the drug-induced deafness gene, including the first double-stranded detection probe for the detection of the C1494T site and the second double-stranded detection probe for the detection of the A1555G site. Detection probes, PCR amplification primers, Taq DNA polymerase, UNG enzyme, dNTP mixture (dATP, dCTP, dGTP and dUTP), 10×PCR buffer (containing Mg 2+ ), negative quality control and positive quality control;
[0087] Among them, the first double-stranded detection probe for detecting the C1494T site is formed by hybridization of P1 and P2. The 5' of P1 is modified with FAM and the 3' is modified with BHQ1. The sequence is shown in SEQ ID NO: 1, and P2 is not modified with fluorescence Group and quenching group, the sequence is as shown in SEQID NO:2;
[0088] The second double-stranded detection probe fo...
Embodiment 2
[0093] Embodiment 2 PCR reaction conditions
[0094] Use the kit of Example 1 to carry out PCR detection on the sample, take out the reagent from the kit and place it at room temperature to melt and oscillate to mix well, then centrifuge at 2000rpm for 10s; set the number of PCR reaction tubes required as n, where n=the number of samples to be tested + 1 tube of negative quality control product + 2 tubes of positive quality control product, take a clean 1.5mL EP tube, mix according to the ratio of 12.5nμL of 2×PCR reaction solution and 10.5nμL of ultrapure water in each tube; The reaction solution was divided into n tubes at 23 μL / tube;
[0095] Add 2 μL each of the sample DNA to be tested, negative quality control DNA, and positive quality control DNA to each PCR reaction tube, close the cap tightly, centrifuge briefly, place in a fluorescent quantitative PCR instrument, and record the order of the samples.
[0096] The reaction conditions were pre-reaction at 50°C for 2 min...
Embodiment 3
[0103] The detection of drug-induced deafness genotype in the clinical sample of embodiment 3
[0104] Using the kit of Example 1, using the reaction system and reaction conditions of Example 2, 100 cases of clinical samples were tested, and all samples were sequenced and verified by the gold standard sequencing method. The test results are shown in Table 3, and the results of statistical analysis and comparison are shown in Table 4.
[0105] Table 3 Occurrence of deafness in clinical samples
[0106]
[0107]
[0108]
[0109]
[0110]
[0111] Table 4 Comparison of test results and sequencing results of the kit of the present invention
[0112]
[0113]
[0114] In Table 4, a positive sequencing result refers to the number of positive genotypes within the detection range of the kit of the present invention or other positive genotypes outside the detection range, and a negative sequencing result refers to the number of negative genotypes within the detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com