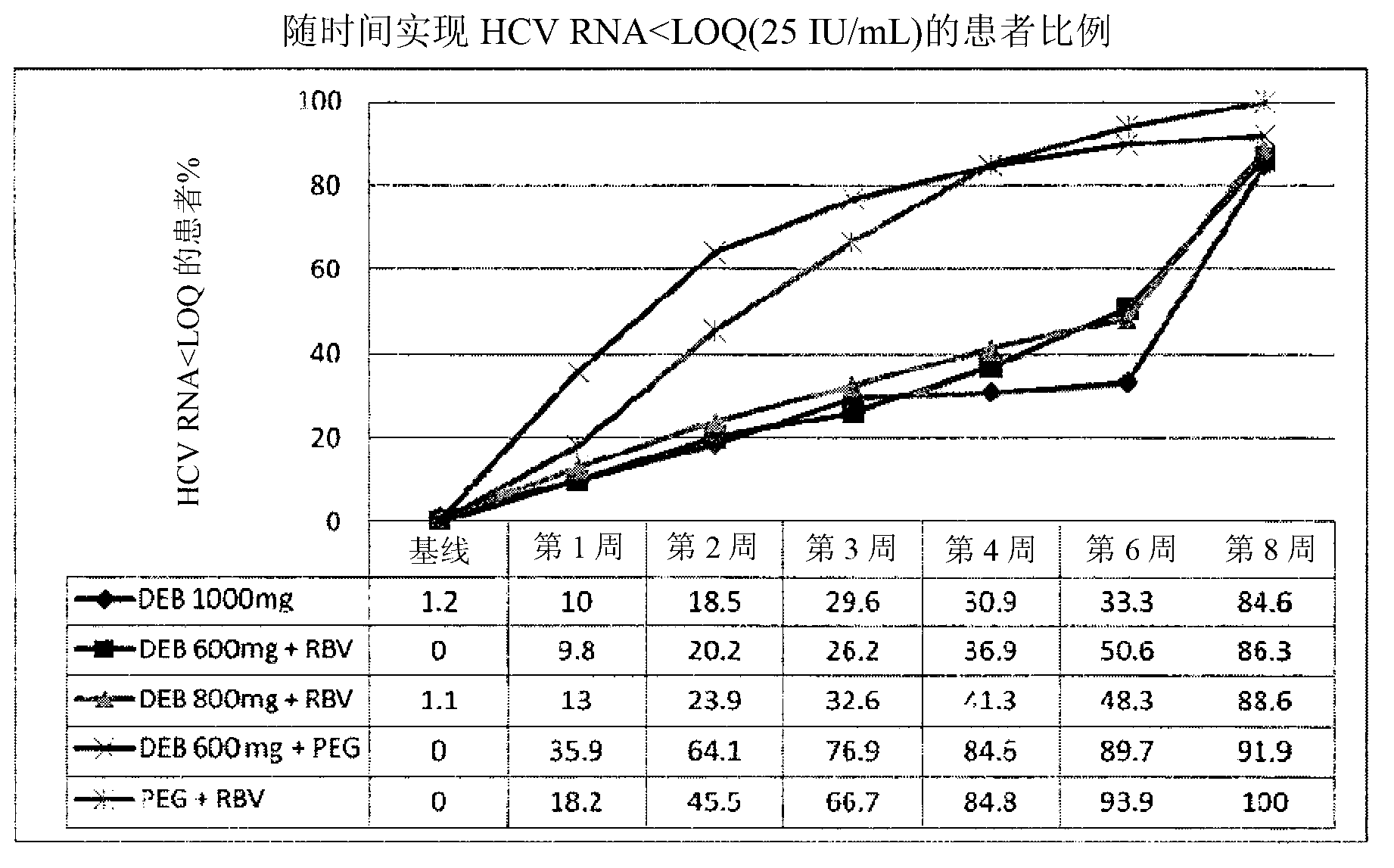
Patents
Literature
36 results about "Hepatitis C virus genotype" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hepatitis C genotype is a type or "strain" of hepatitis C virus. There are 6 genotypes of hepatitis C around the world. In the United States, 3 genotypes are common: Genotype 1. Genotype 2. Genotype 3.
Determination of hepatitis C virus genotype
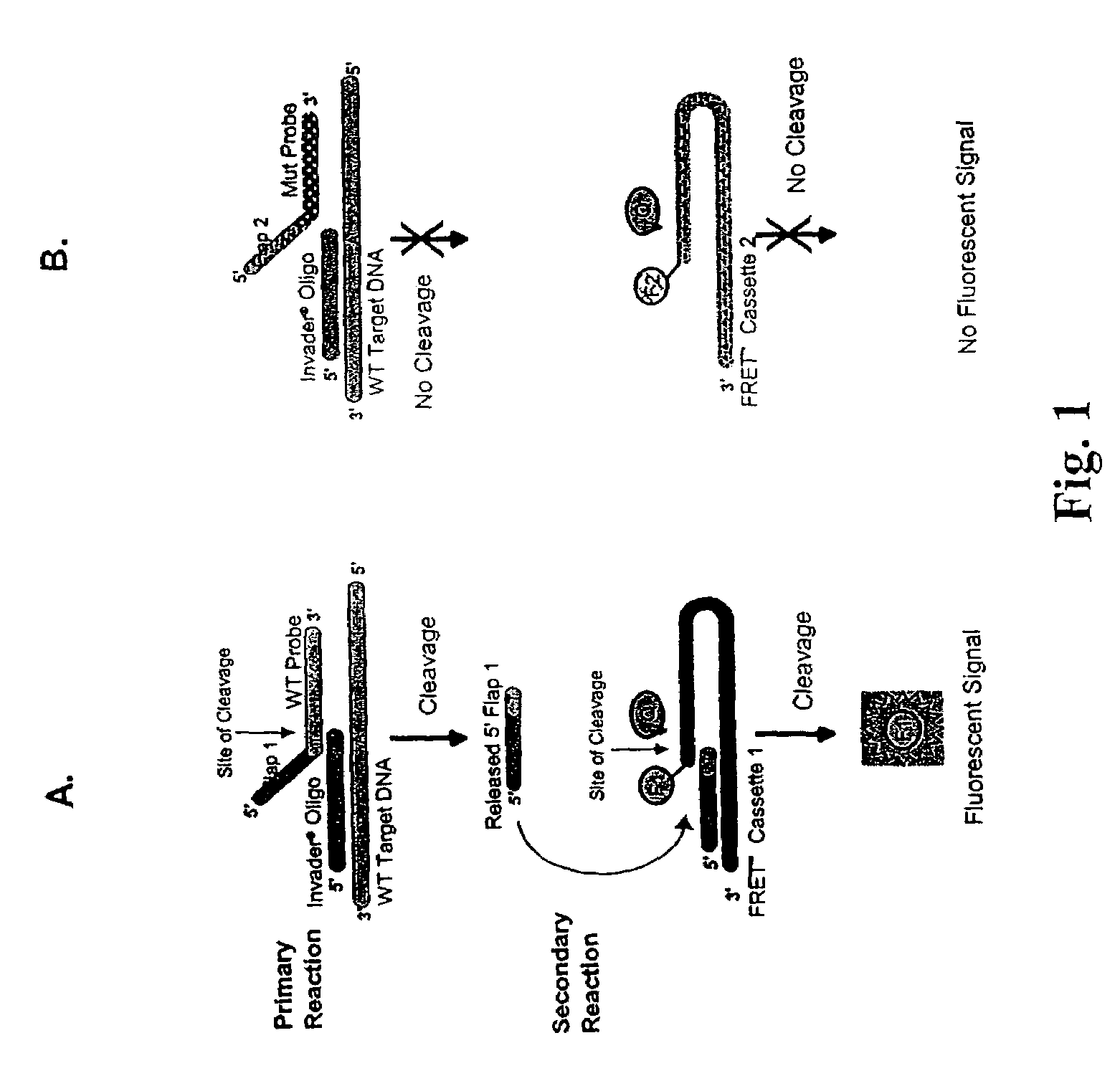
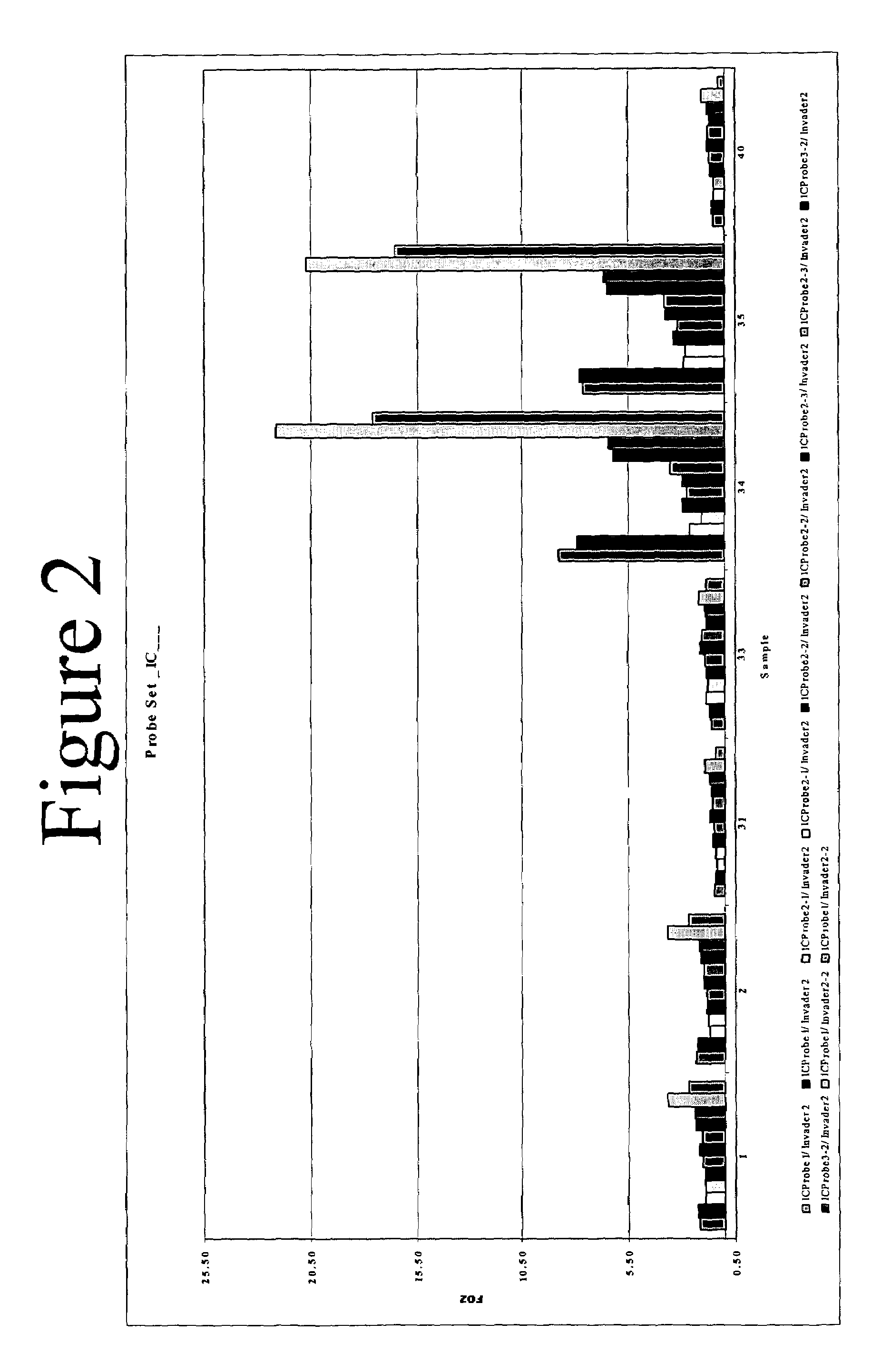
The present invention provides compositions and methods for the detection and characterization of HCV sequences. More particularly, the present invention provides compositions, methods and kits for using invasive cleavage structure assays (e.g. the INVADER assay) to screen nucleic acid samples, e.g., from patients, to determine HCV genotype.
Owner:GEN PROBE INC
Kit for detecting genotyping and IL28-site polymorphism of hepatitis C virus (HCV)
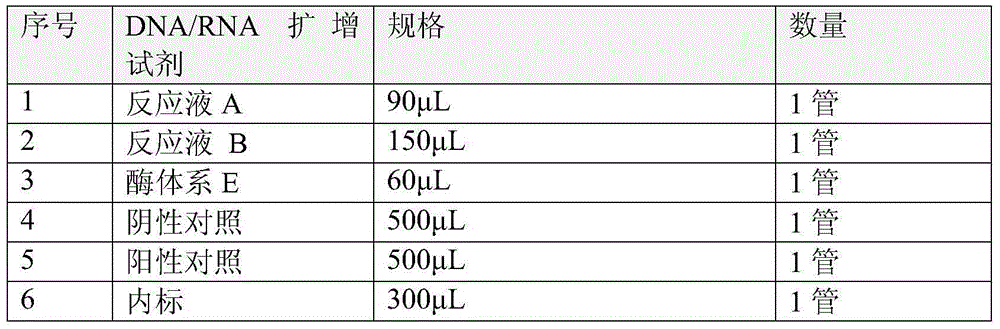
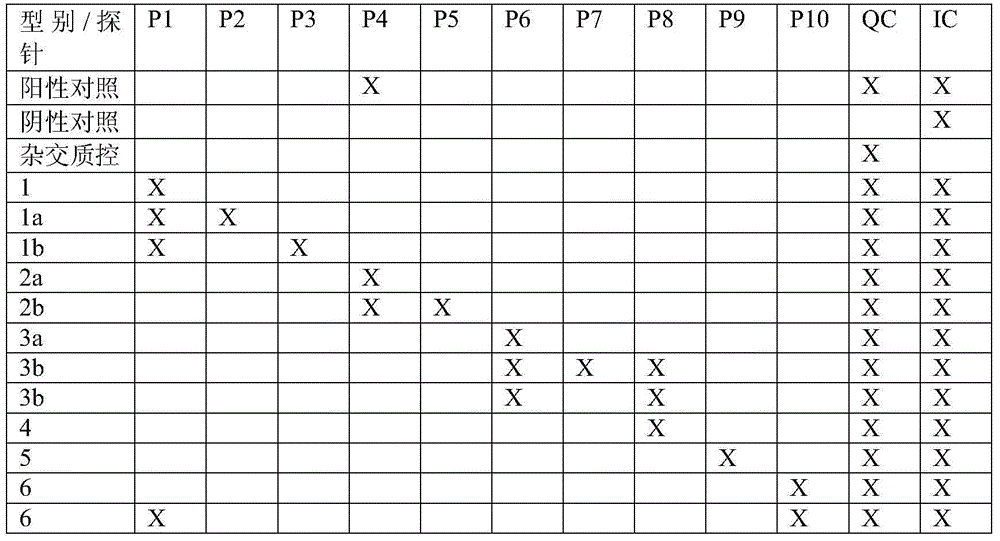
The invention relates to a kit for detecting the genotype and IL28-site polymorphism of a hepatitis C virus (HCV), in particular to a kit for detecting the genotype and IL28-site polymorphism of an HCV, which is prepared by using a nucleic acid reverse dot hybridization technology. The kit comprises an amplification reagent, a low-density chip using a nylon membrane as a carrier and a hybridization reagent, and can simultaneously detect the genotype of the HCV and the IL28B gene polymorphism closely related to the HCV treatment, thereby providing a more reliable basis for the individual hepatitis C treatment.
Owner:DAAN GENE CO LTD
Efficient cell culture system for hepatitis C virus genotype 5A
Owner:HVIDOVRE HOSPITAL
HCV gene typing detecting reagent kit
InactiveCN101377486AEasy to operateMicrobiological testing/measurementBiological testingGene typeHepatitis C virus genotype
The invention relates to a kit for detecting a hepatitis c virus genotype, particularly relates to that the nucleic acid reverse dot blot hybridization technology is used to prepare a kit for hepatitis c virus genotype detection. The invention is used for rapidly and accurately distinguishing the hepatitis c virus genotype in a clinical blood sample.
Owner:DAAN GENE CO LTD
Efficient cell culture system for hepatitis c virus genotype 1a and 1b
The present inventors developed hepatitis C virus 1a / 2a and 1b / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and NS2 were replaced by the corresponding genes of the genotype Ia reference strain H77C or TN or the corresponding genes of the genotype Ib reference strain J4. Sequence analysis of recovered 1a / 2a and 1b / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in e.g. p7, NS2 and / or NS3. In addition, the inventors demonstrate the possibility of using adaptive mutations identified for one HCV isolate in generating efficient cell culture systems for other isolates by transfer of mutations across isolates, subtypes or major genotypes. Furthermore neutralization studies showed that viruses of e.g. genotype 1 were efficiently neutralized by genotype Ia, 4a and 5a serum, an effect that could be utilized e.g. in vaccine development and immunological prophylaxis. The inventors in addition demonstrate the use of the developed systems for screening of antiviral substances in vitro and functional studies of the virus, e.g. identification of receptors required for HCV entry
Owner:HVIDOVRE HOSPITAL
Efficient cell culture system for hepatitis c virus genotype 6a
InactiveUS20110059512A1Raise the potentialSsRNA viruses positive-senseSugar derivativesSequence analysisHCV Genotyping
The present inventors developed hepatitis C virus 6a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and the complete NS2 were replaced by the corresponding genes of the genotype 6a reference strain HK6a. Sequence analysis of recovered 6a / 2a recombinants from 2 transfection experiments and subsequent reverse genetic studies revealed adaptive mutations in E1 and E2. Conclusion: The developed 6a / 2a viruses provide a robust in vitro tool for research in HCV genotype 6, including vaccine studies and functional analyses.
Owner:HVIDOVRE HOSPITAL
EFFICIENT CELL CULTURE SYSTEM FOR HEPATITIS C VIRUS GENOTYPE 7a
InactiveUS20110294195A1High infectivity titerEfficient growth processSsRNA viruses positive-senseBacteriaSequence analysisHepatitis C virus genotype
Genotype 7a has been identified recently, thus not much is known about the biology of this new, major HCV genotype. The present inventors developed hepatitis C virus 7a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and the complete NS2 were replaced by the corresponding genes of the genotype 7a strain QC69 and characterized them in Huh7.5 cells. Sequence analysis of 7a / JFH1 recombinants recovered after viral passage in Huh7.5 cells following 4 independent transfection experiments revealed adaptive mutations in Core, E2, NS2, NS5A and NS5B. In reverse genetic studies the importance of these mutations for improved growth kinetics was shown. Adapted 7a / JFH1 viruses showed growth kinetics, infectivity and RNA titers comparable to a previously developed 3a / JFH1 reference virus. Conclusion: The developed 7a / JFH1 viruses provide a robust in vitro tool for research in HCV genotype 7, including vaccine studies and functional analyses.
Owner:HVIDOVRE HOSPITAL
Efficient cell culture system for hepatitis c virus genotype 5a
InactiveUS20110021611A1Organic active ingredientsSsRNA viruses positive-senseSequence analysisSerial passage
The present inventors developed 5a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and all of or part of NS2 were replaced by the corresponding genes of the genotype 5a reference strain SA13. Compared to the J6 / JFH control virus, after transfection of in vitro transcripts in Huh7.5 cells, production of infectious viruses was delayed. However, in subsequent viral passages efficient spread of infection and HCV RNA titers as high as for J6 / JFH were obtained. Infectivity titers were at all time points analyzed comparable to J6 / JFH control virus. Sequence analysis of recovered 5a / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in p7, NS2 and / or NS3. Infectivity of the 5a / 2a viruses was CD81 and SR-BI dependant, and the recombinant viruses could be neutralized by chronic phase sera from patients infected with genotype 5a. Conclusion: The developed 5a / 2a viruses provide a robust in vitro tool for research in HCV genotype 5, including vaccine studies and functional analyses of an increasingly important genotype in South Africa and Europe.
Owner:HVIDOVRE HOSPITAL
Efficient cell culture system for hepatitis C virus genotype 6A
The present inventors developed hepatitis C virus 6a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and the complete NS2 were replaced by the corresponding genes of the genotype 6a reference strain HK6a. Sequence analysis of recovered 6a / 2a recombinants from 2 transfection experiments and subsequent reverse genetic studies revealed adaptive mutations in E1 and E2. Conclusion: The developed 6a / 2a viruses provide a robust in vitro tool for research in HCV genotype 6, including vaccine studies and functional analysis.
Owner:HVIDOVRE HOSPITAL
Efficient cell culture system for hepatitis C virus genotype 7a
Owner:HVIDOVRE HOSPITAL
Kit for HCV virus genotyping detection, use method and application thereof
InactiveCN105986038AImprove anti-interference abilityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesHybridization probeFluorescence
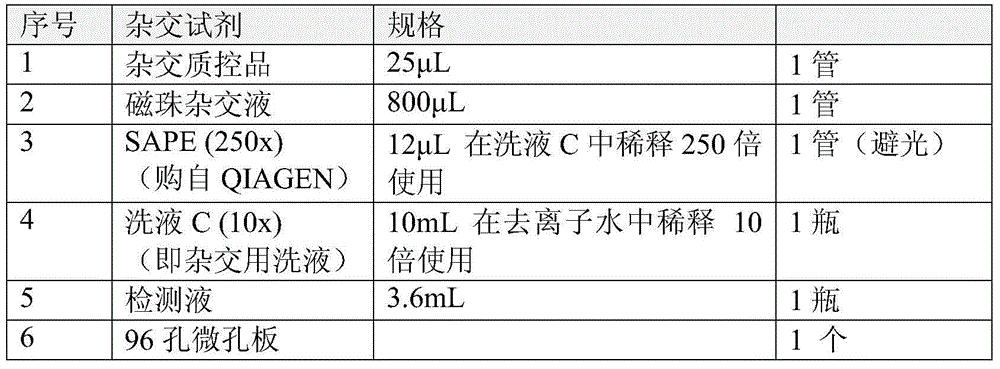
The invention discloses a kit for HCV virus genotyping detection, a use method and an application thereof. The kit includes a nucleic acid amplification reagent and a hybridization reagent. The nucleic acid amplification reagent includes primers represented as the SEQ ID No. 1-4. The hybridization reagent includes probes represented as the SEQ ID No. 5-16. The use method of the kit includes the steps of: 1) nucleic acid extraction of a sample; 2) preparation of the amplification reagent; 3) PCR amplification; 4) a hybridization reaction; 5) a fluorescent reaction; 6) detection; and 7) determination on the genotype of HCV virus according to fluorescent signal value in the detection reaction system. The kit can be used for detecting nine different genotypes of HCV virus, has high flux, high sensitivity and high type coverage area, allows multi-target simultaneous detection and is simple in operation.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
HCV (Hepatitis c virus) genotype detection kit
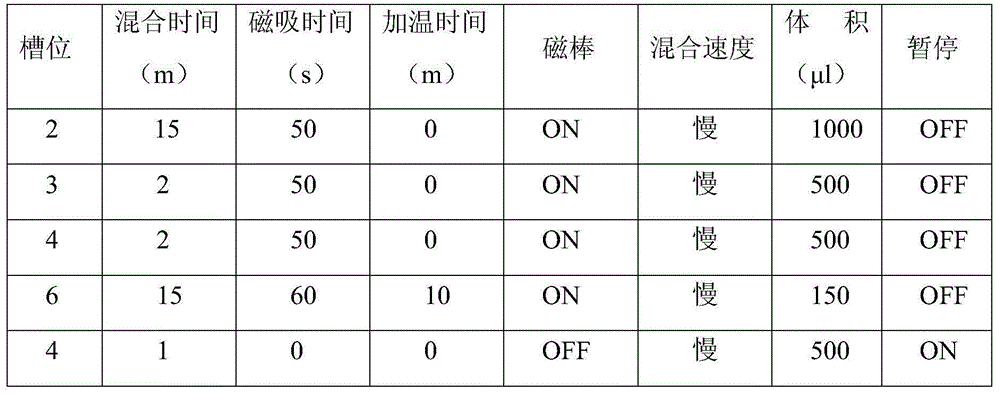
ActiveCN103710464AStrong specificityHigh purityMicrobiological testing/measurementMicroorganism based processesMagnetic beadFluorescence
The invention relates to a HCV (hepatitis c virus) genotype detection kit. The kit extracts a sample nucleic acid by using a magnetic bead method; by using a real-time fluorescence quantitative PCR (polymerase chain reaction) technology, and taking a highly conserved area of a HCV genome as an amplification target, a specific primer and a TaqMan probe are designed, then HCV genes are subjected to rapid and accurate genotyping detection on a real-time fluorescence PCR instrument by PCR amplification, and an internal standard is added in a system, so that false negative results can be effectively prevented.
Owner:SANSURE BIOTECH INC
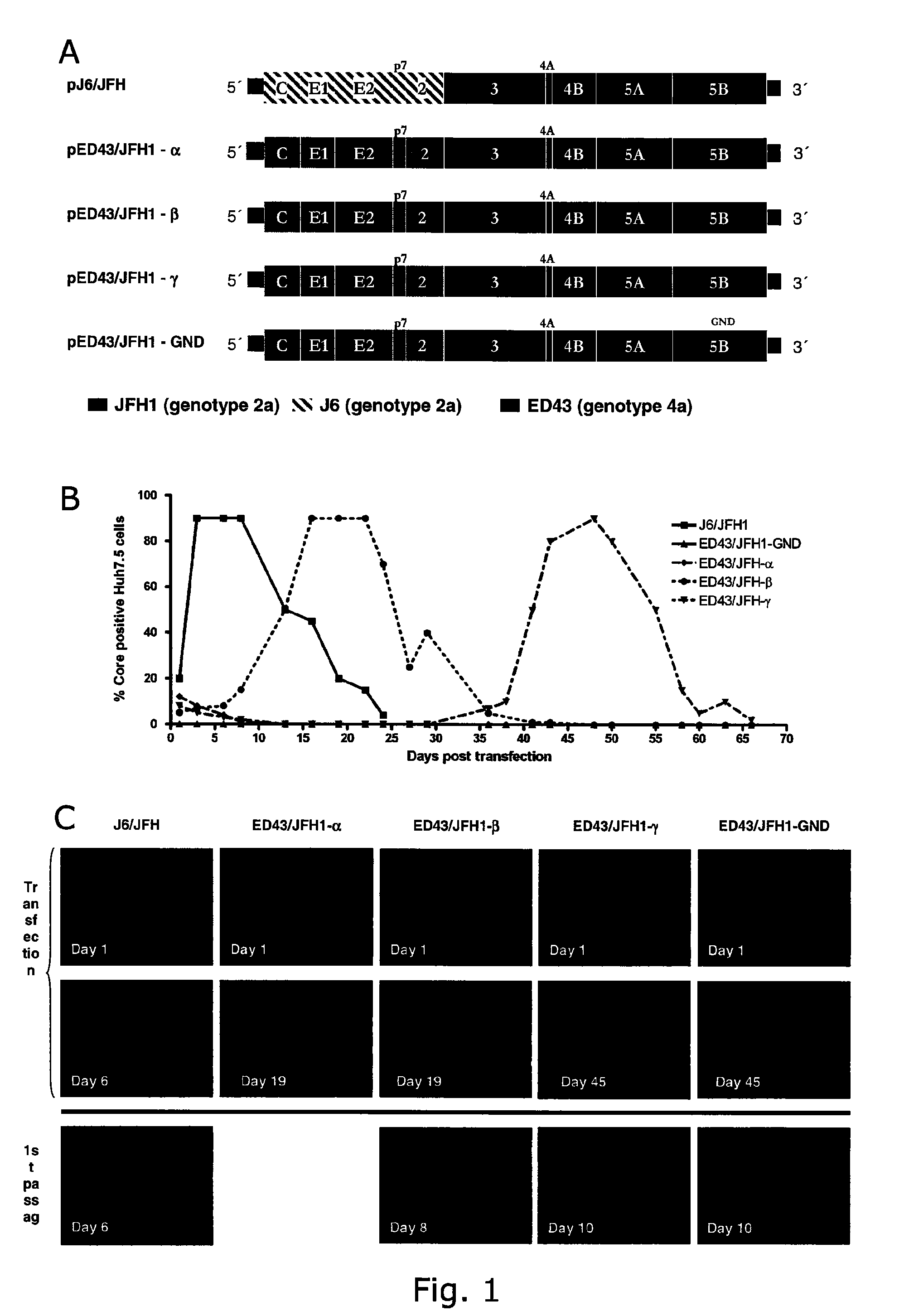
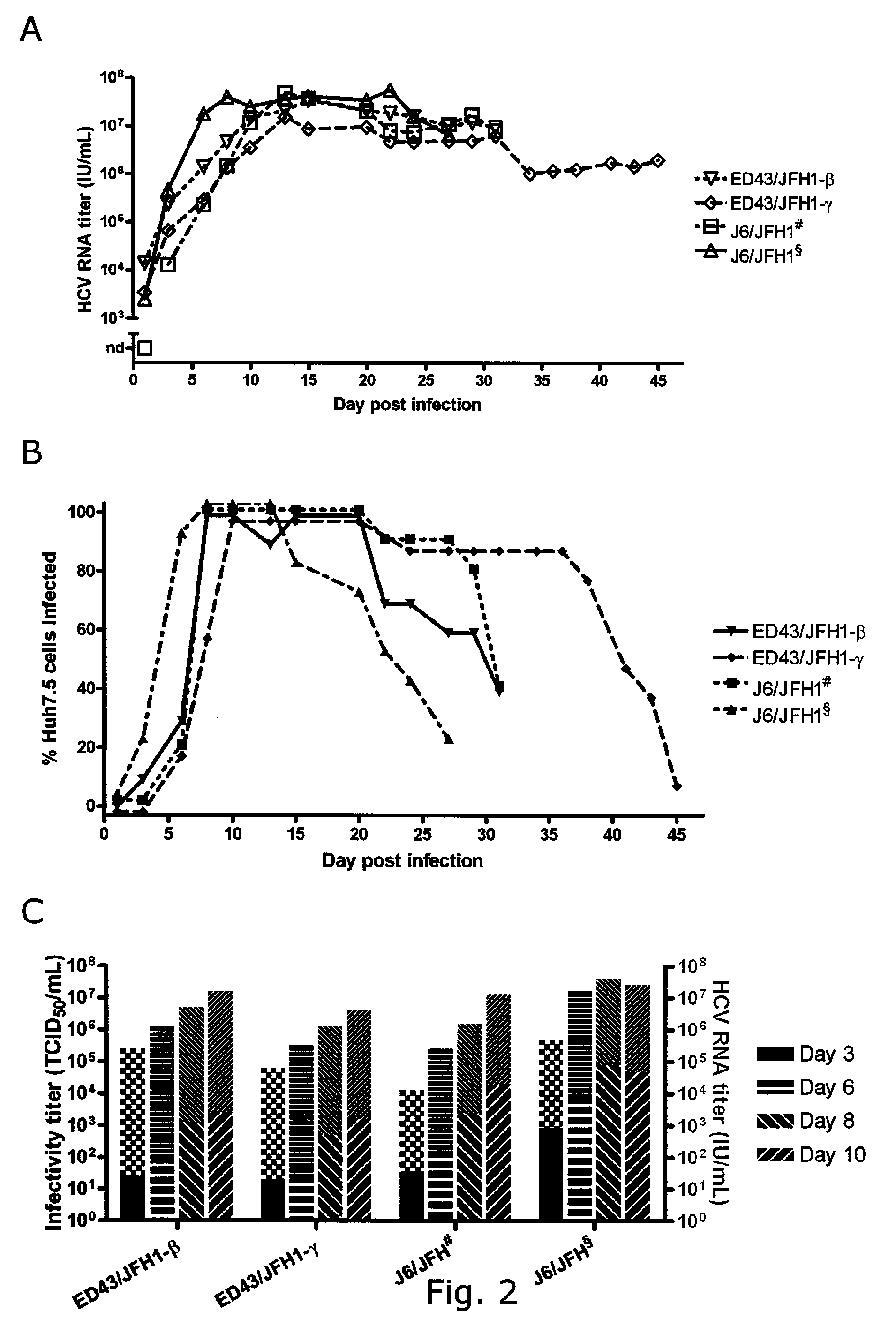
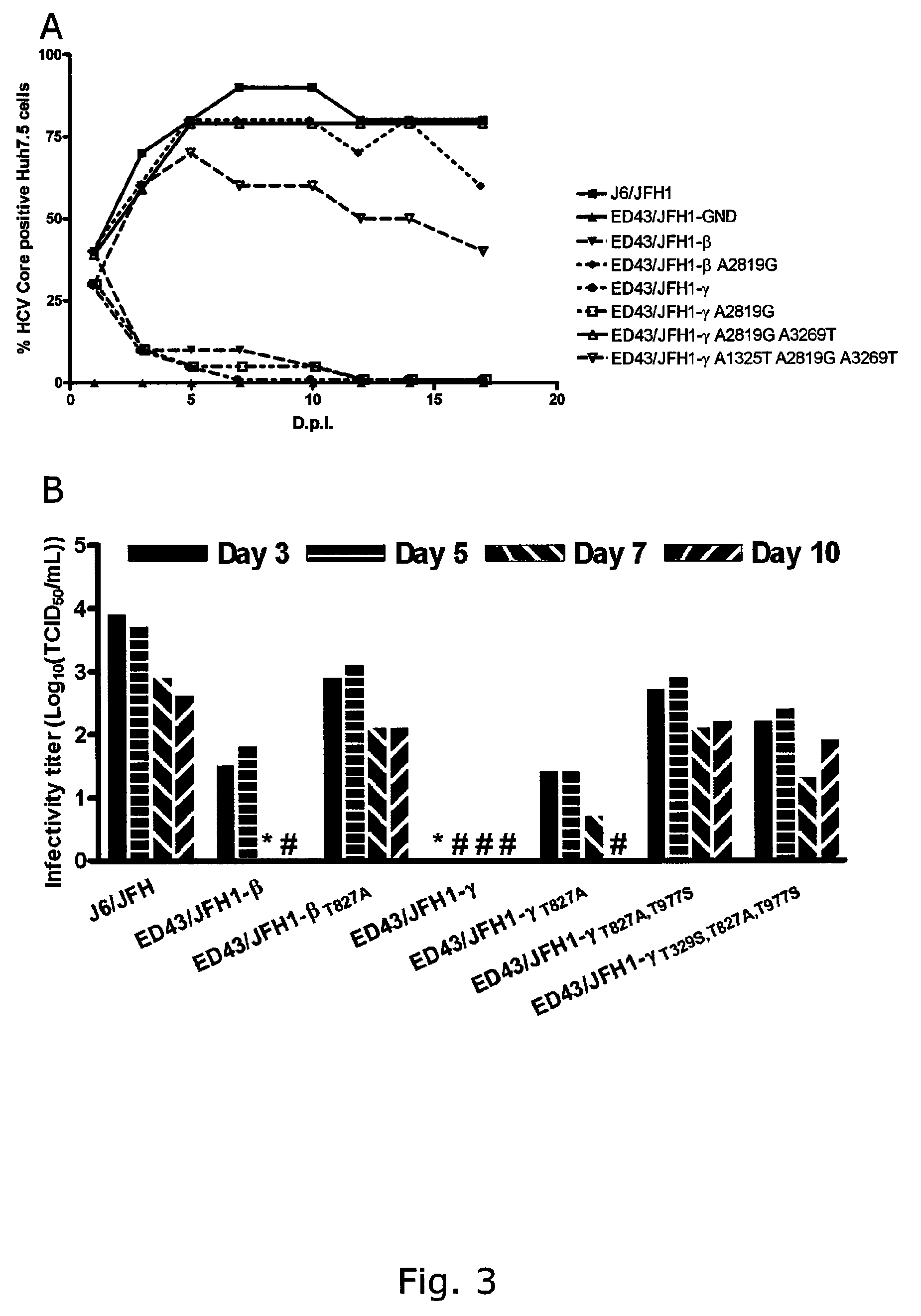
Adaptive mutations allow establishment of JFH1-based cell culture systems for hepatitis C virus genotype 4A
The present inventors developed three 4a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and all of or part of NS2 were replaced by the corresponding genes of the genotype 4a reference strain ED43. The 4a / 2a junction in NS2 was placed after the first transmembrane domain (α), in the cytoplasmic part (β) or at the NS2 / NS3 cleavage site (y). Following transfection of Huh7.5 cells with RNA transcripts, infectious viruses were produced in the ED43 / JFH1-β and -y cultures only. Compared to the 2a control virus, production of infectious viruses was significantly delayed. However, in subsequent passages efficient spread of infection and high HCV RNA titers were obtained. Infectivity titers were approximately 10-fold lower than for the 2a control virus. Sequence analysis of recovered 4a / 2a recombinants from 3 serial passages and subsequent reverse genetic studies revealed a vital dependence on a mutation in the NS2 4a part. ED43 / JFH1-γ further depended on a second NS2 mutation. Infectivity of the 4a / 2a viruses was CD81 dependent. Conclusion: The developed 4a / 2a viruses provide a robust in vitro tool for research in HCV genotype 4, including vaccine studies and functional analyses of an increasingly important genotype in the Middle East and Europe.
Owner:HVIDOVRE HOSPITAL
Efficient cell culture system for hepatitis C virus genotype 1A and 1B
The present inventors developed hepatitis C virus 1a / 2a and 1b / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and NS2 were replaced by the corresponding genes of the genotype Ia reference strain H77C or TN or the corresponding genes of the genotype Ib reference strain J4. Sequence analysis of recovered 1a / 2a and 1b / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in e.g. p7, NS2 and / or NS3. In addition, the inventors demonstrate the possibility of using adaptive mutations identified for one HCV isolate in generating efficient cell culture systems for other isolates by transfer of mutations across isolates, subtypes or major genotypes. Furthermore neutralization studies showed that viruses of e.g. genotype 1 were efficiently neutralized by genotype Ia, 4a and 5a serum, an effect that could be utilized e.g. in vaccine development and immunological prophylaxis. The inventors in addition demonstrate the use of the developed systems for screening of antiviral substances in vitro and functional studies of the virus, e.g. identification of receptors required for HCV entry.
Owner:HVIDOVRE HOSPITAL
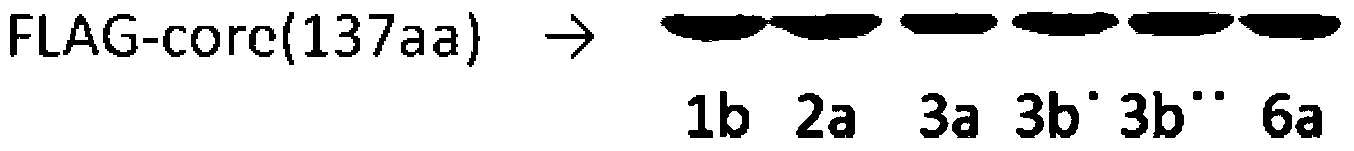
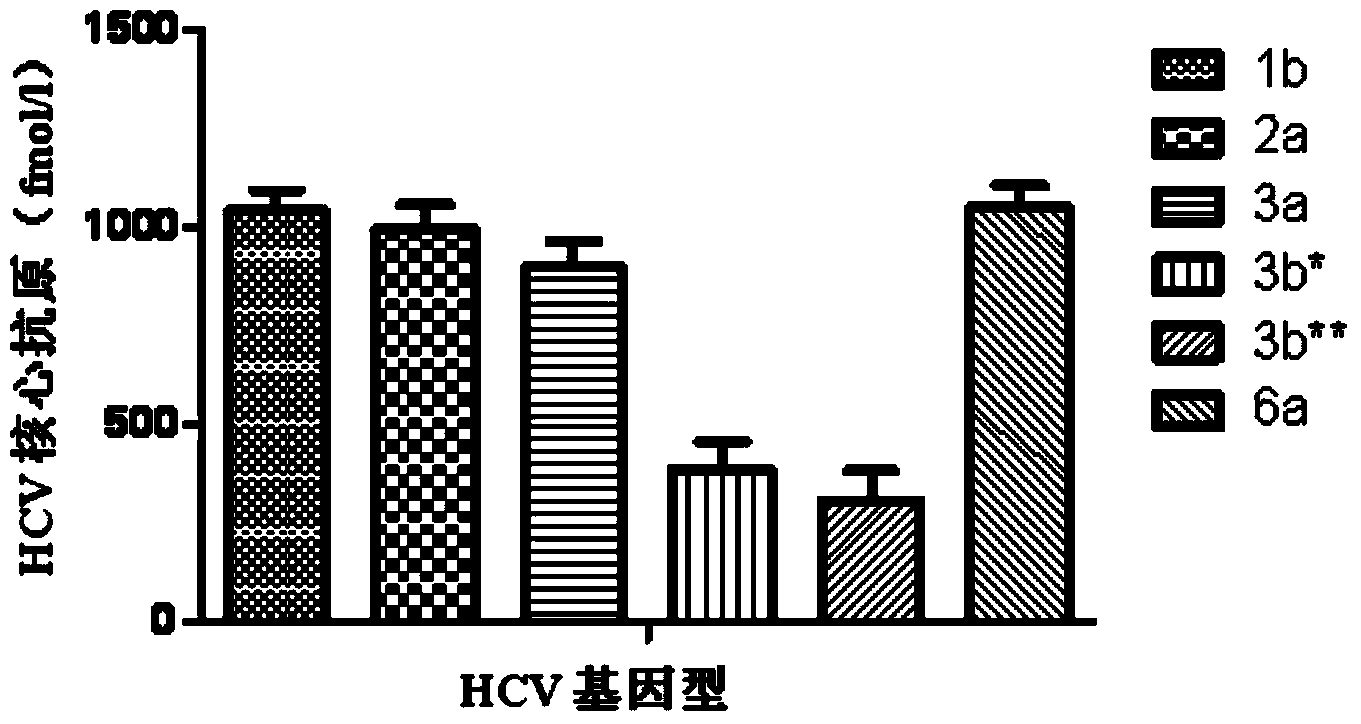
Core antigen fragment for detecting hepatitis C virus and application of core antigen fragment
InactiveCN104374915AMake up for lack of sensitivityImprove detection sensitivity and accuracyBiological material analysisHCV GenotypingAntigen levels
The invention provides a core antigen fragment for detecting hepatitis C virus and an application of the core antigen fragment. The antigen level of different HCV genotype serum samples is detected by using an Abbott Architect HCV quantitative core antigen kit, the sensitivity of different genotypes is compared and detected, a sample of specific genotype which influences the detection sensitivity is analyzed, a polymorphic site of the core antigen which influences the detection sensitivity of the kit is found, and the amino acid sequence of the core antigen is as shown in SEQ ID NO.1-2. The amino acid fragment of the polymorphic site of the core antigen can serve as a molecular marker of HCV core antigen detection and is used for detecting the genotype of hepatitis C virus 3b. Moreover, the existence of the molecular marker can indicate that the sensitivity of the conventional quantitative antigen detection kit is reduced when the kit is used for detecting a sample of the genotype of hepatitis C virus 3b, normalization and standardization of the core antigen detection can be facilitated, and the core antigen fragment can be used for preparing a kit for detecting the HCV 3b genotype.
Owner:PEOPLES HOSPITAL PEKING UNIV
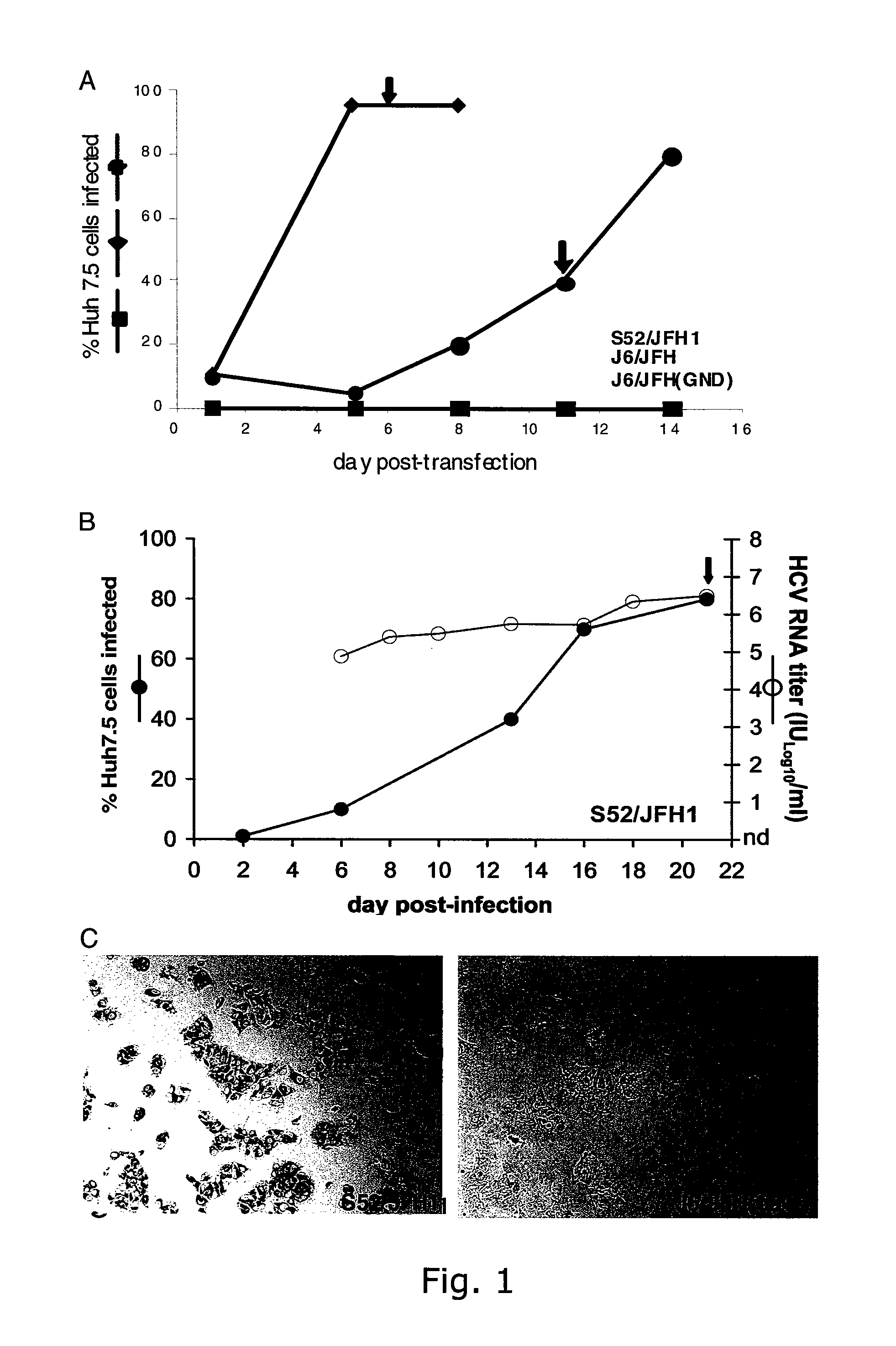
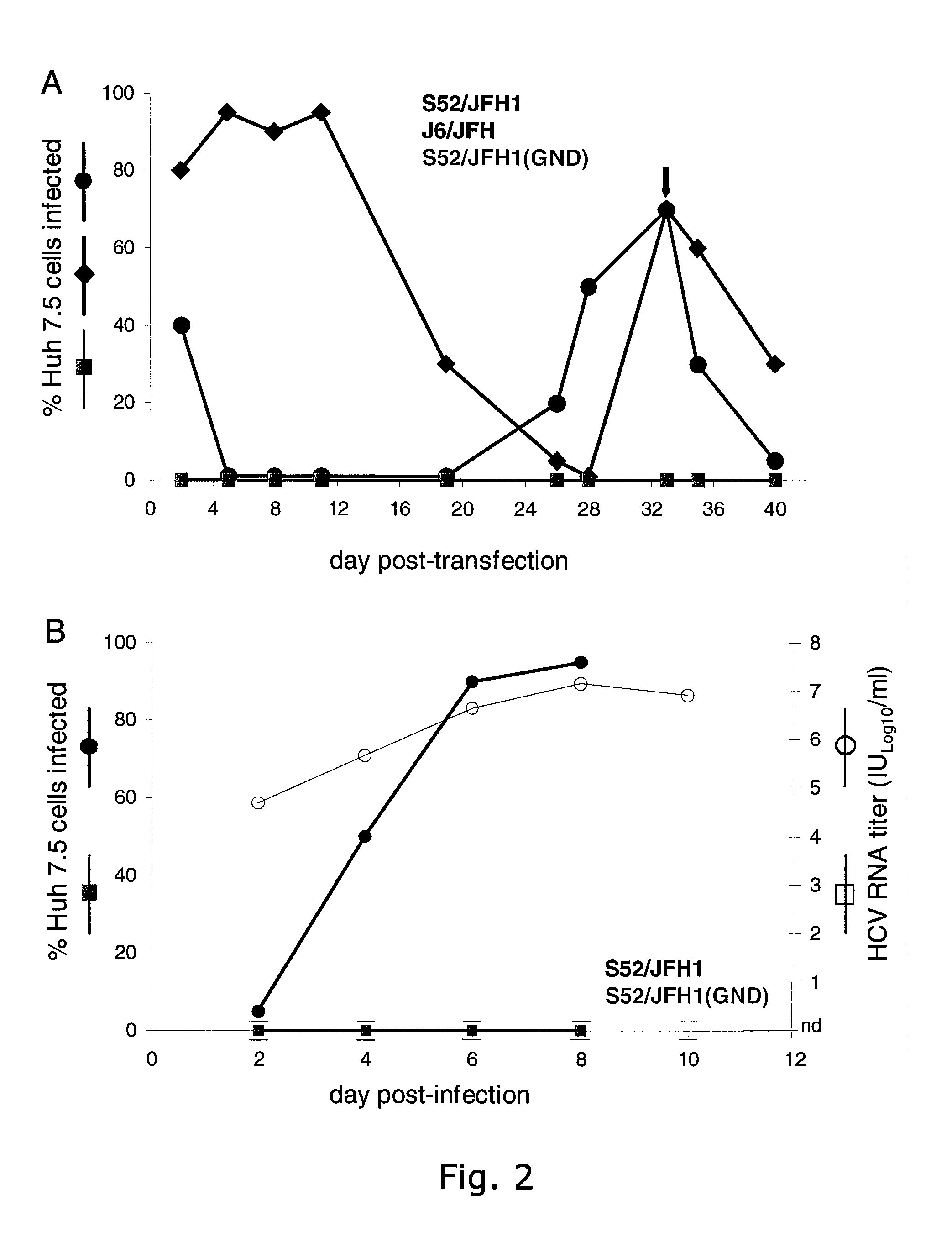
Cell culture system of a hepatitis C genotype 3a and 2a chimera
InactiveUS8945584B2SsRNA viruses positive-senseSugar derivativesHepatitis C virus genotypeHepatitis viral c
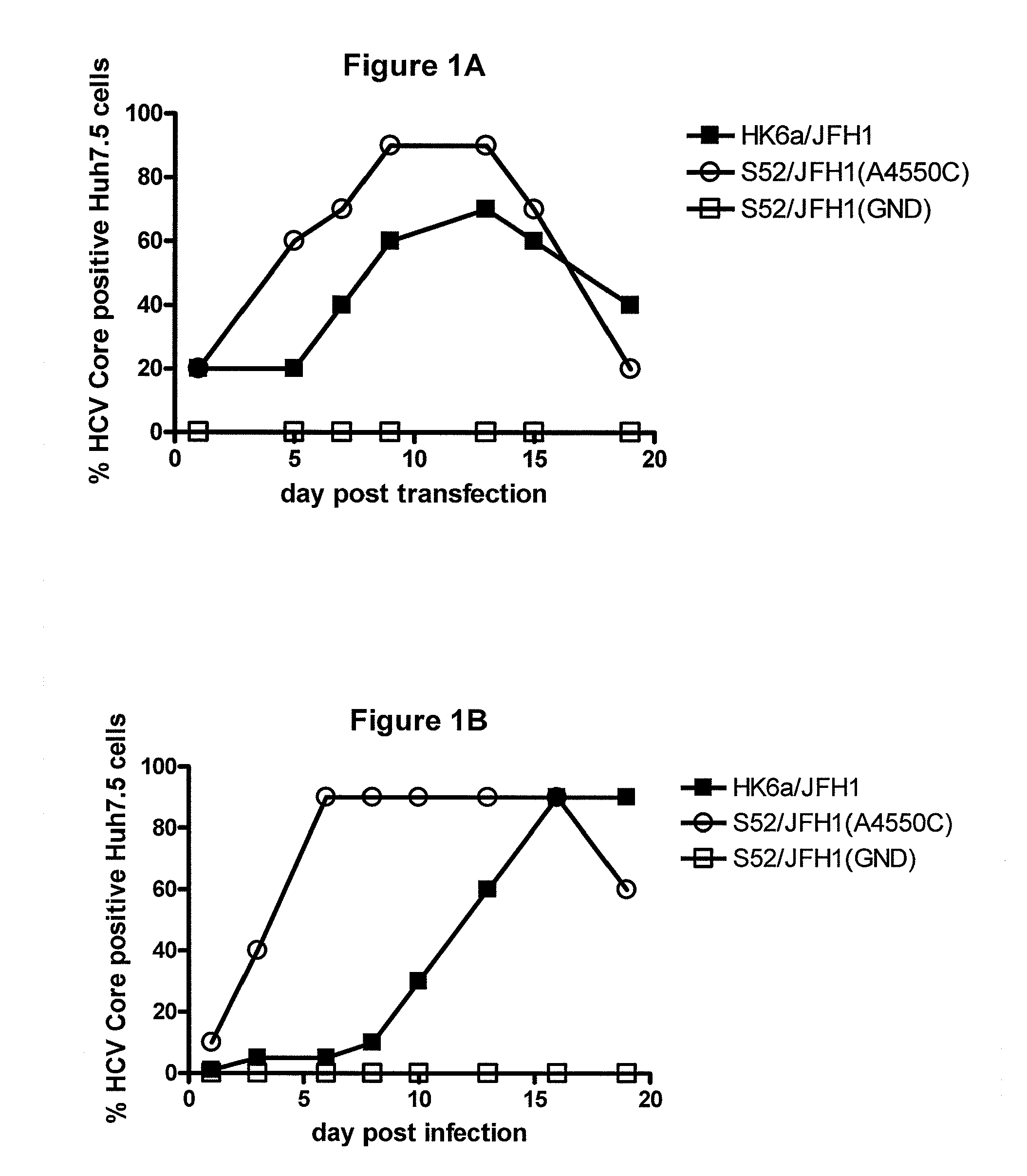
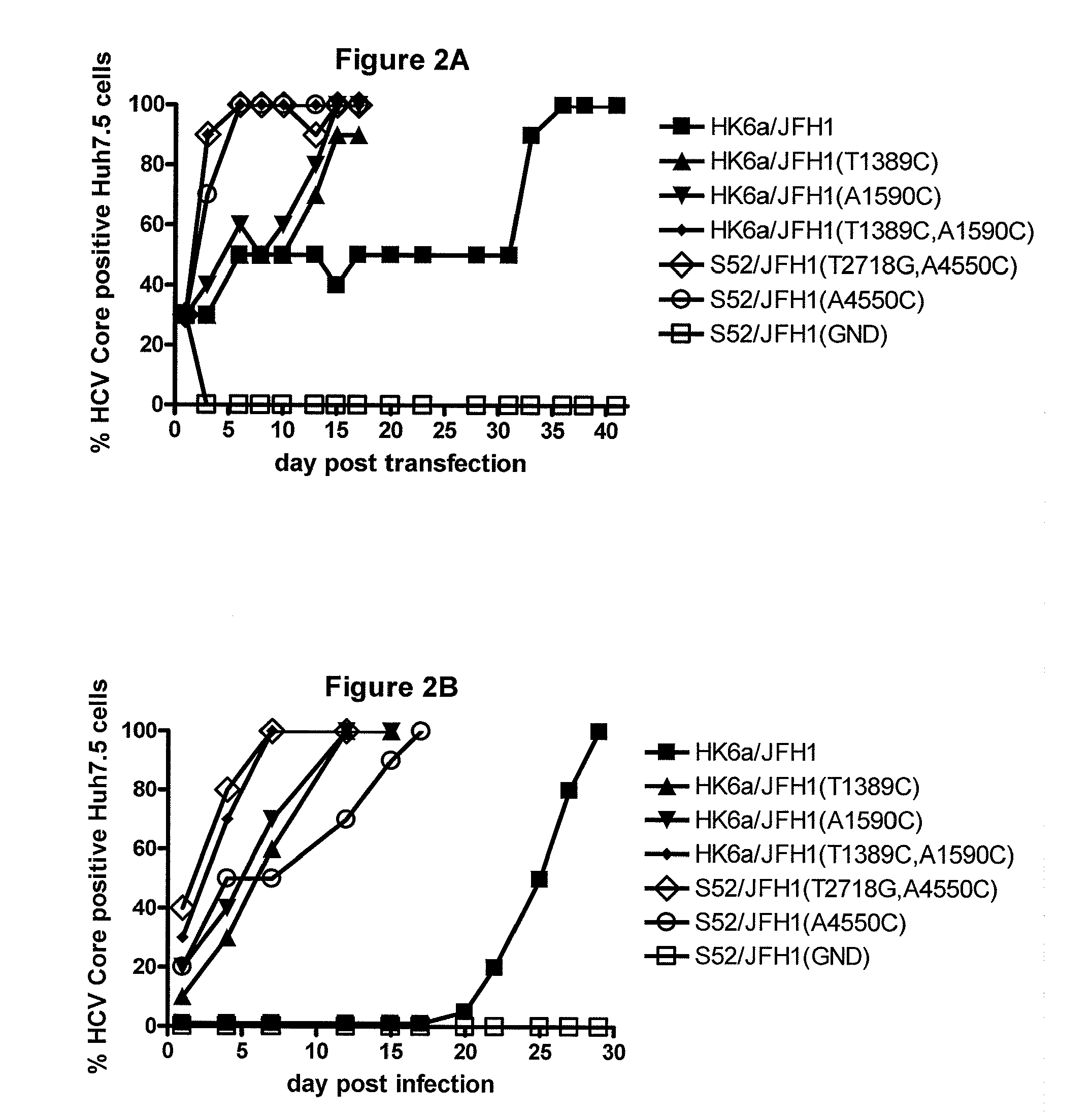
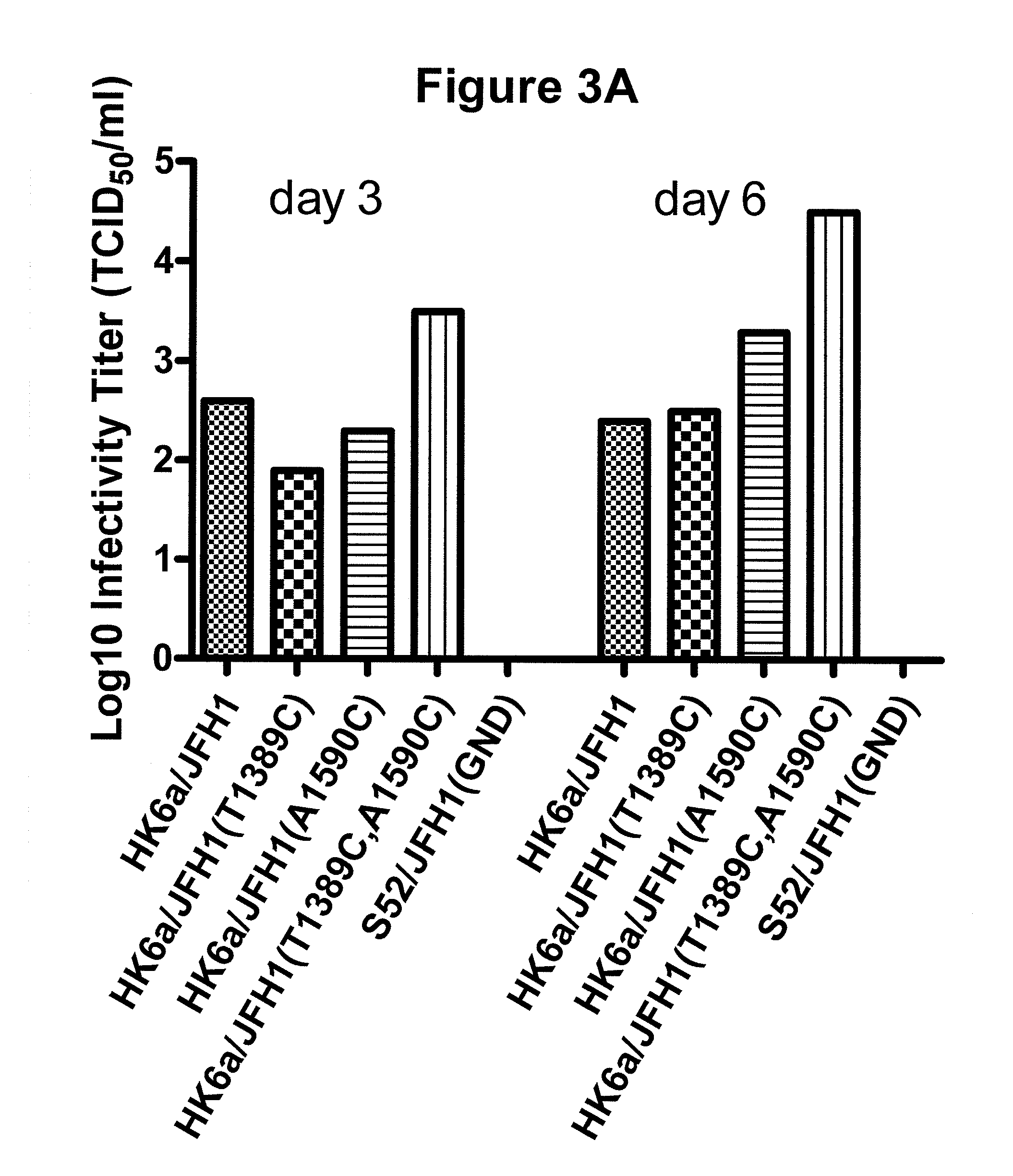
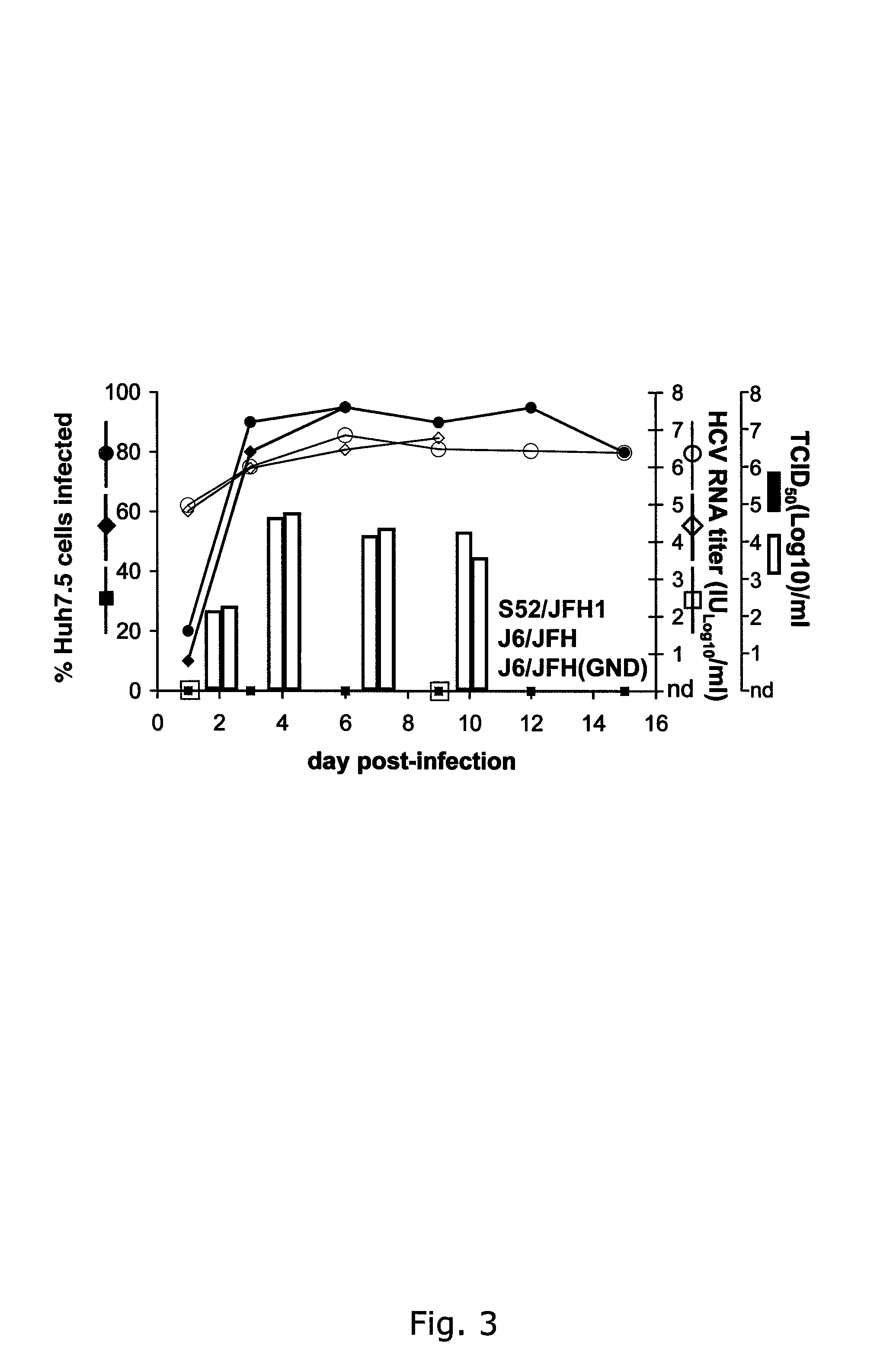
A robust and genetically stable cell culture system for Hepatitis C Virus (HCV) genotype 3a is provided. A genotype 3a / 2a (S52 / JFH1) recombinant containing the structural genes (Core, E1, E2), p7 and NS2 of strain S52 was constructed and characterized in Huh7.5 cells. S52 / JFH1 and J6 / JFH viruses passaged in cell culture had comparable growth kinetics and yielded similar peak HCV RNA titers and infectivity titers. Direct genome sequencing of cell culture derived S52 / JFH1 viruses identified putative adaptive mutations in Core, E2, p7, NS3, and NS5A; clonal analysis revealed that all genomes analyzed exhibited different combinations of these mutations. Finally, viruses resulting from transfection with RNA transcripts of five S52 / JFH1 recombinants containing these combinations of putative adaptive mutations performed as efficiently as J6 / JFH viruses in Huh7.5 cells and were all genetically stable after viral passage.
Owner:HVIDOVRE HOSPITAL
Efficient cell culture system for hepatitis C virus genotype 2B
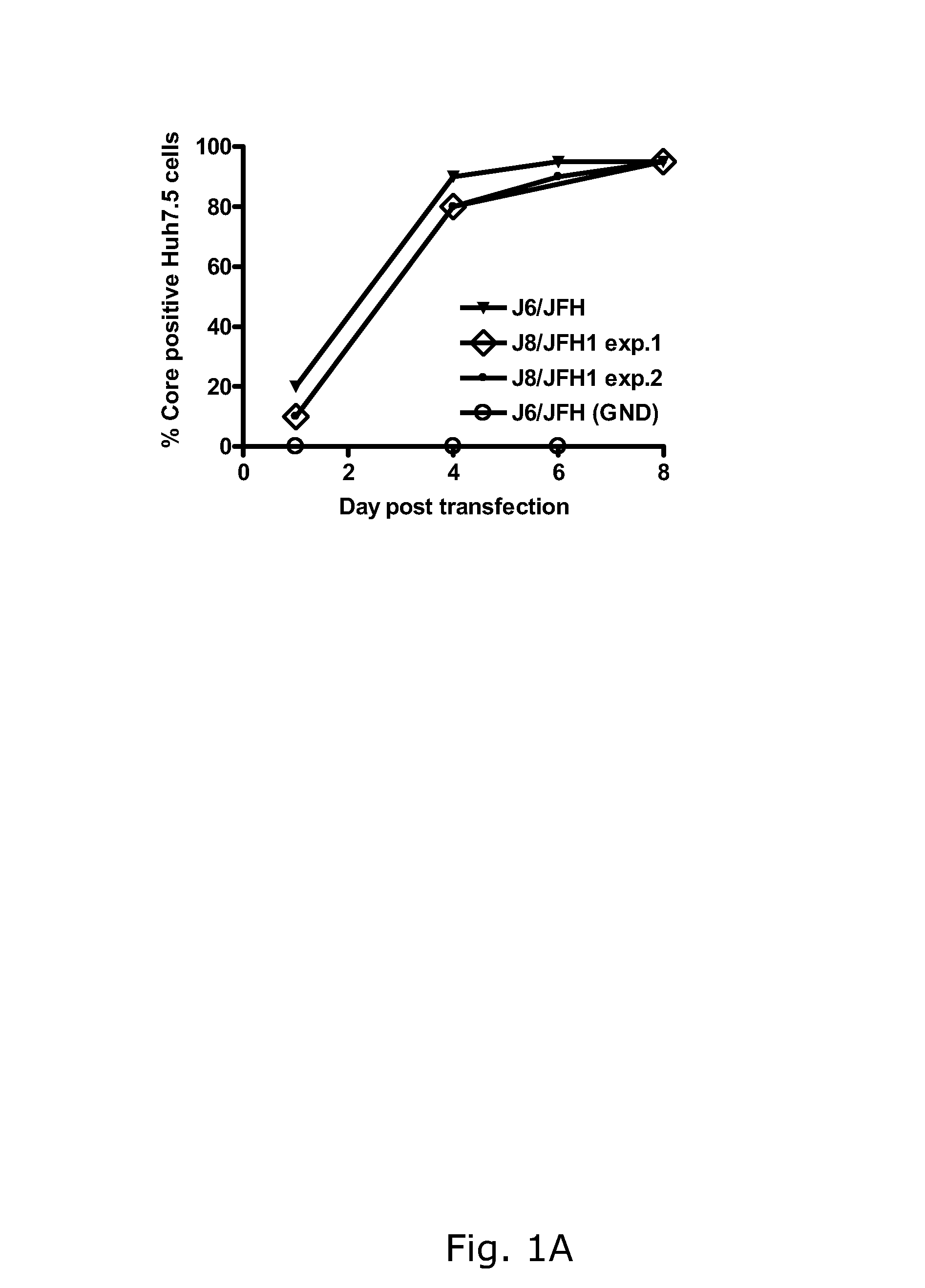
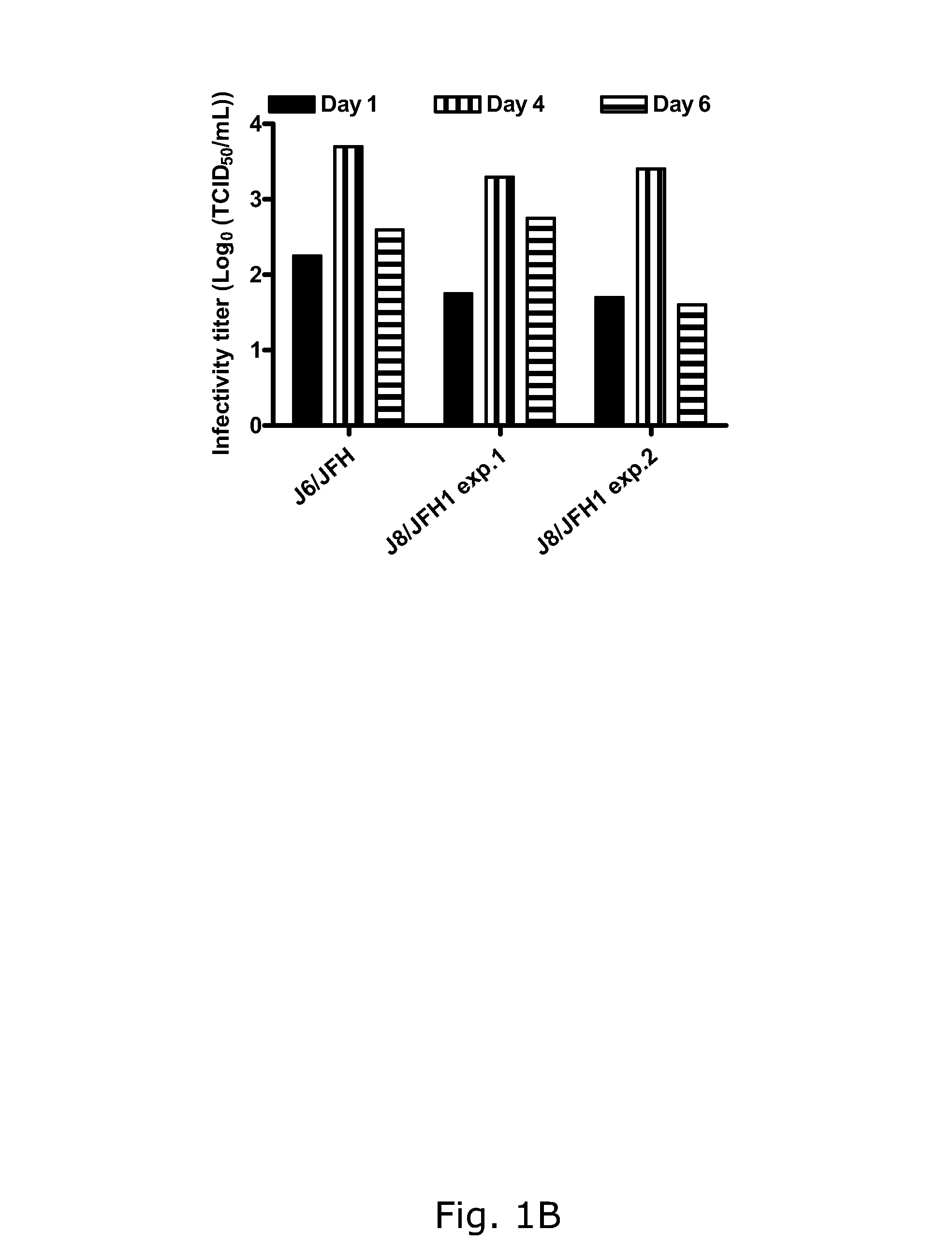
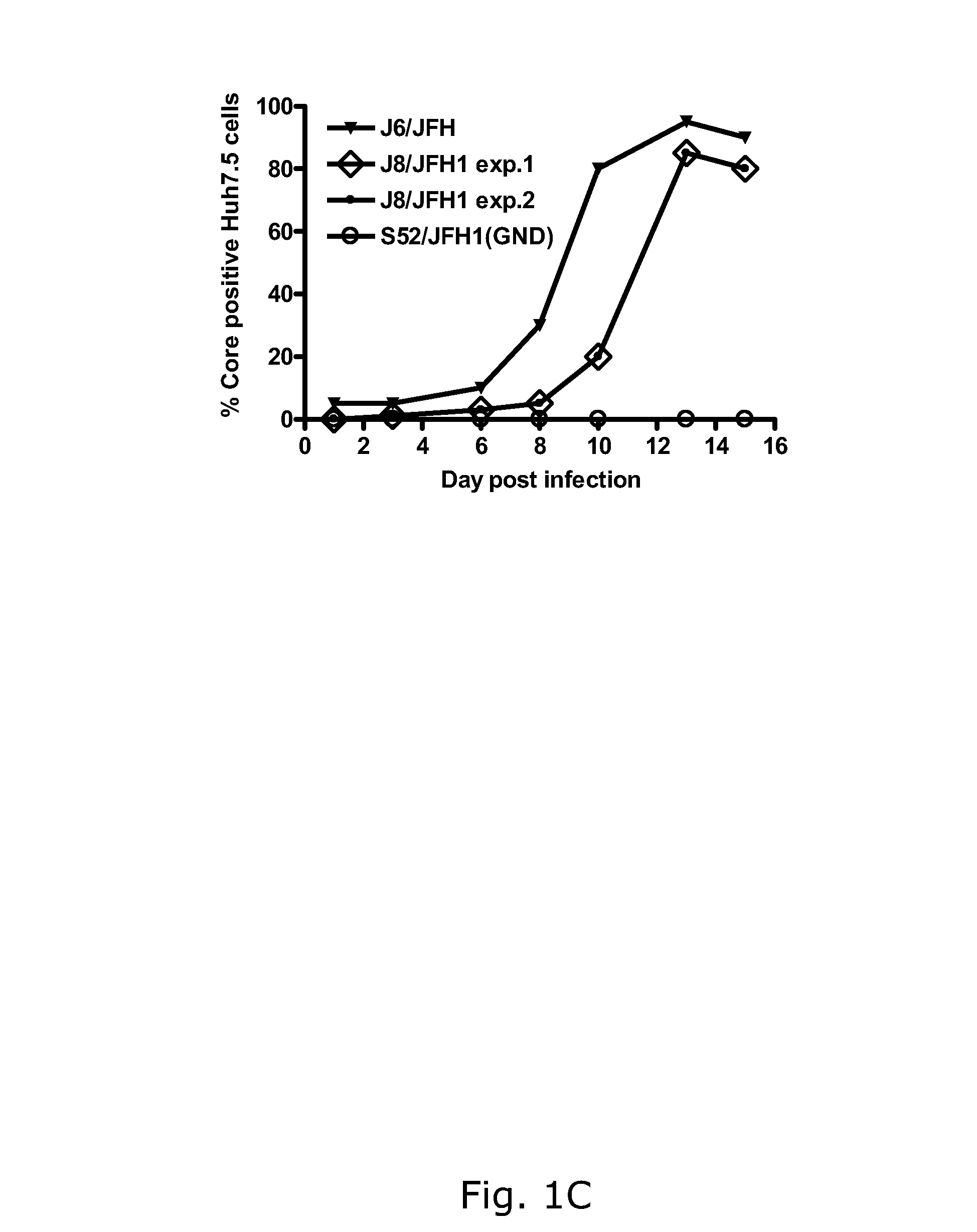
The present inventors developed hepatitis C virus 2b / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and the complete NS2 were replaced by the corresponding genes of the genotype 2b reference strain J8. Sequence analysis of recovered 2b / 2a recombinants from 2 transfection experiments revealed that 2b / 2a was genetically stable. Conclusion: The developed 2b / 2a viruses provide a robust in vitro tool for research in HCV genotype 2b, including vaccine studies and functional analysis.
Owner:HVIDOVRE HOSPITAL
Hepatitis c virus genotype detection kit
ActiveCN103725797AMicrobiological testing/measurementMicroorganism based processesFluorescenceHepatitis C virus genotype
The invention relates to a hepatitis c virus (HCV) genotype detection kit. The kit utilizes a paramagnetic particle method to extract sample nucleic acid, and by adopting a real-time fluorescence quantitative PCR (polymerase chain reaction) technology, taking a highly conserved area of an HCV genome as an amplification target, and designing a specificity primer and a TaqMan probe, typing detection of the HCV gene can be rapidly and accurately carried out on a real-time fluorescence PCR instrument through PCR amplification.
Owner:SANSURE BIOTECH INC
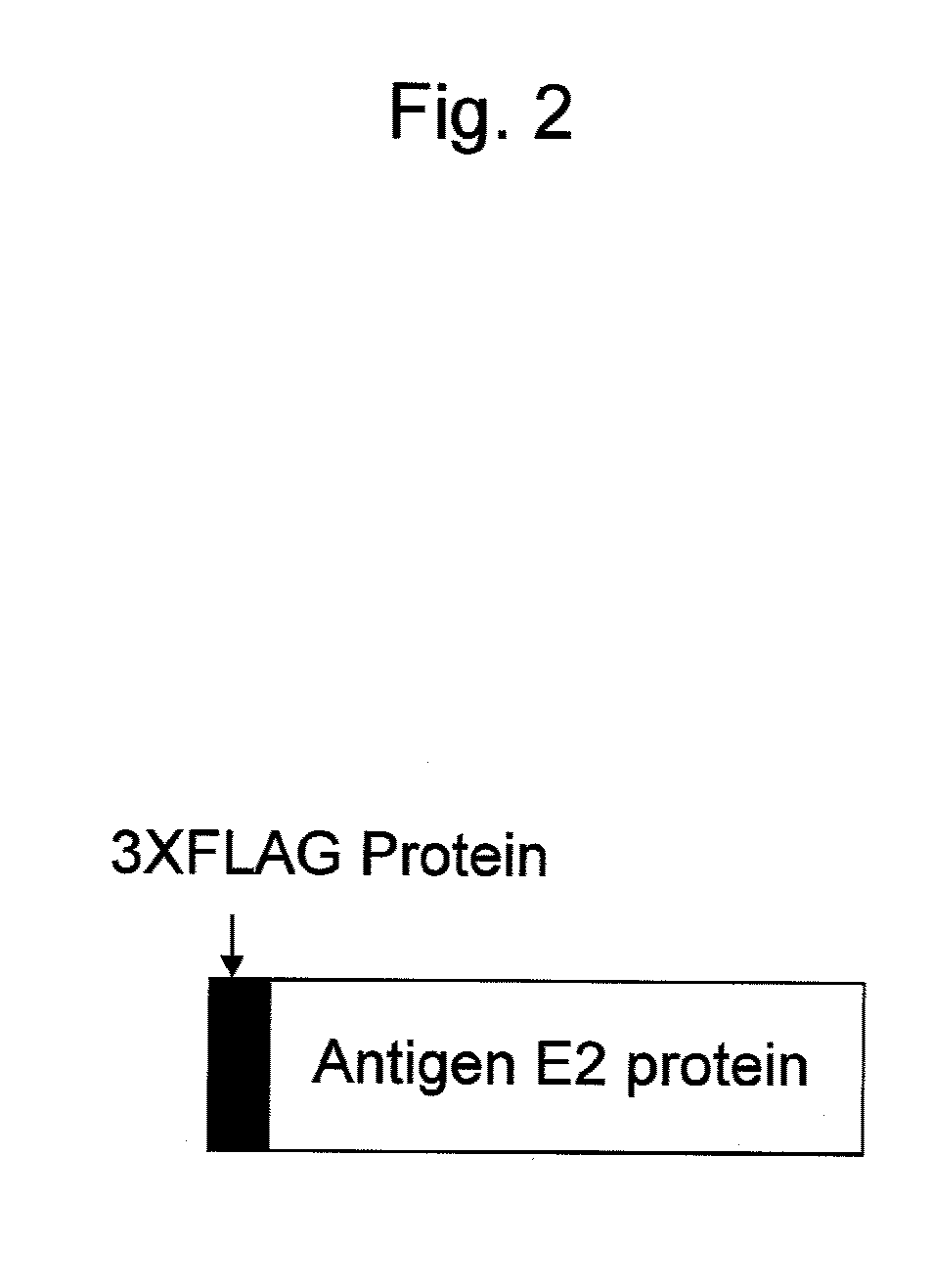
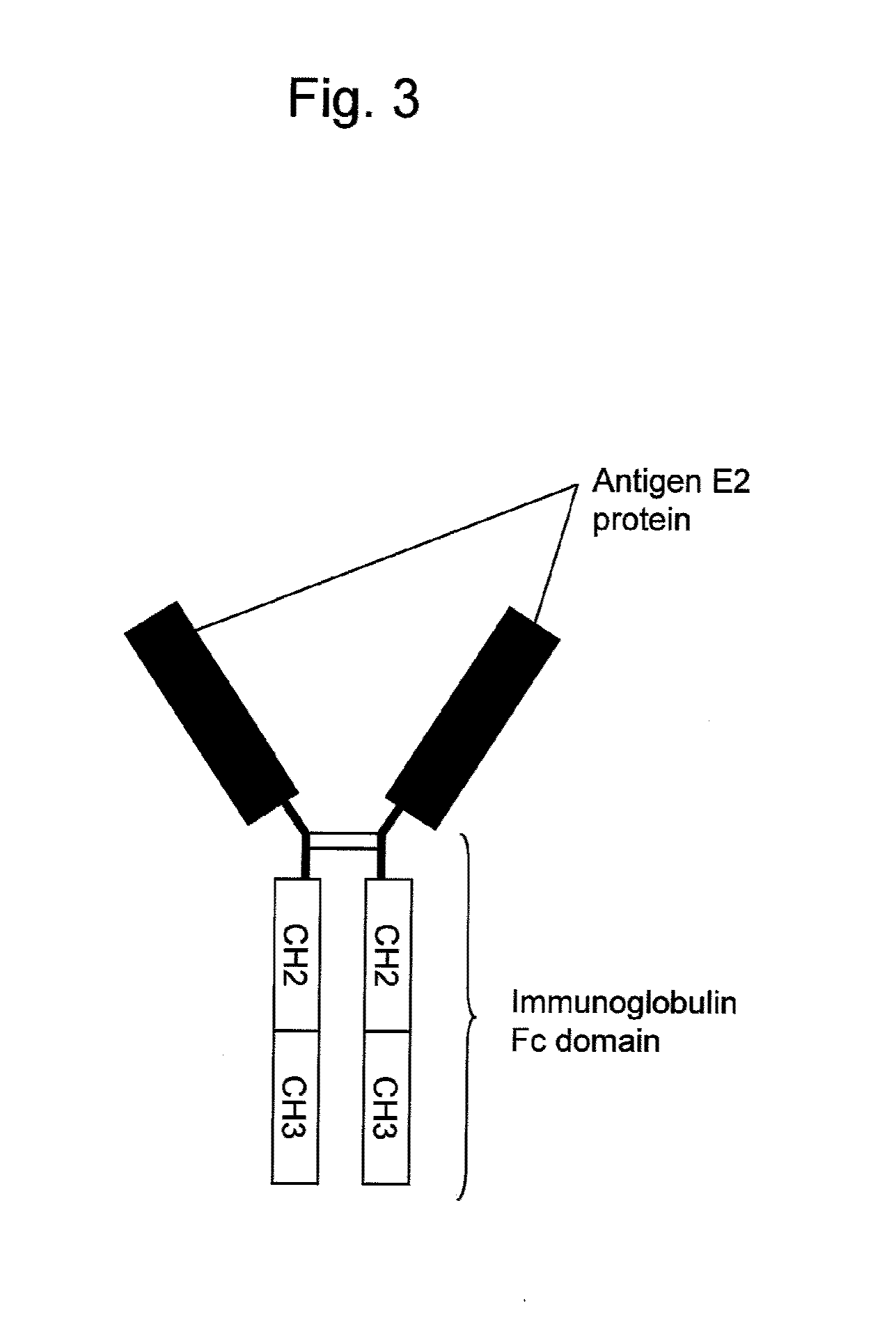
Antibody binding to envelope protein 2 of hepatitis c virus and method for identifying genotype of hepatitis c virus using the same
InactiveUS20110201014A1Reduce adverse reactionsValid choiceMicrobiological testing/measurementBiological material analysisHepatitis C virus genotypeHepacivirus
Owner:TORAY IND INC +1
Kit for HCV virus genotyping detection and SNP locus detection of IL28B, use method and application thereof
InactiveCN105986040AImprove anti-interference abilityReduce dosageMicrobiological testing/measurementMicroorganism based processesFluorescenceHybridization reaction
The invention discloses a kit for HCV virus genotyping detection and SNP locus detection of IL28B, a use method and an application thereof. The kit includes a nucleic acid amplification reagent and a hybridization reagent. The use method of the kit includes the steps of: 1) nucleic acid extraction of a sample; 2) preparation of the amplification reagent; 3) PCR amplification; 4) a hybridization reaction; 5) a fluorescent reaction; 6) detection; and 7) determination on the genotype of HCV virus and the genotype of an SNP locus in the IL28B gene. The kit can be used for detecting nine different HCV viruses and meanwhile detecting the genotypes of two loca in the IL28B gene which relates to treatment effect on the HCV. The kit has high flux, high sensitivity and high type coverage area, allows multi-target simultaneous detection and is simple in operation.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
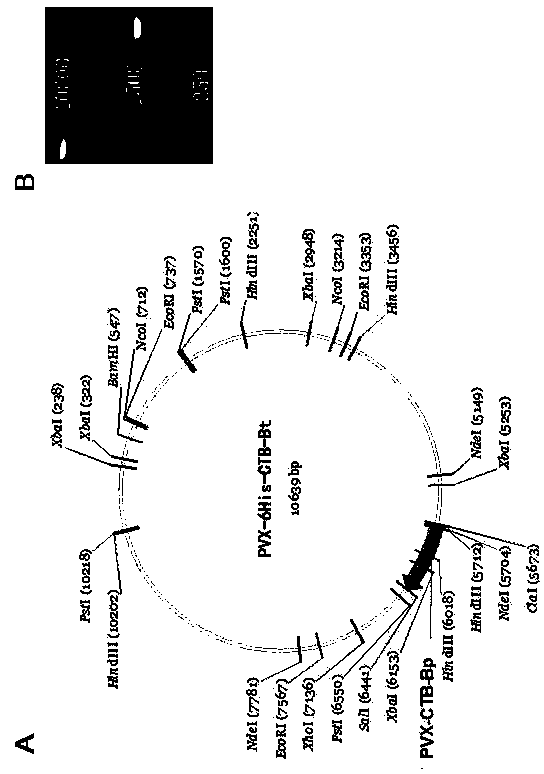
Expression vector PVX-6His-CTBt-Bt for producing multi-epitope vaccine of hepatitis c virus
InactiveCN104353071ADetermining the inhibitory effectAntiviralsAntibody medical ingredientsHepatitis c viralCellular antigens
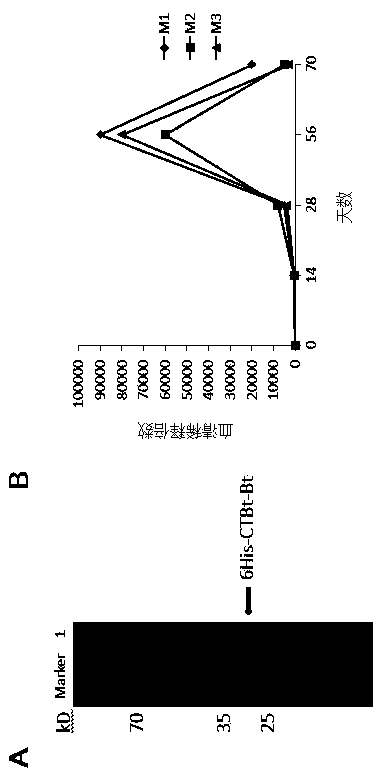
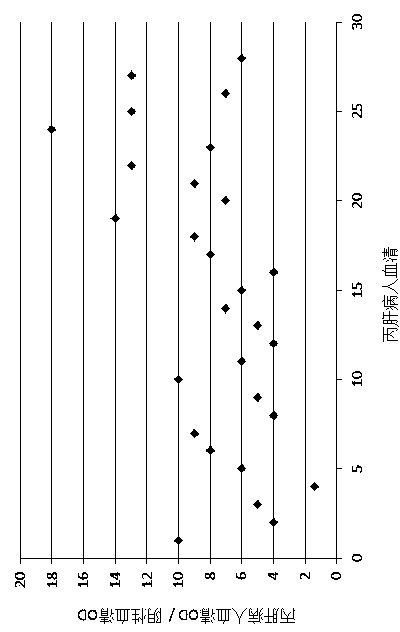
The invention discloses an expression vector PVX-6His-CTBt-Bt for producing a multi-epitope vaccine of hepatitis c virus. The multi-epitope vaccine 6His-CTBt-Bt of hepatitis c virus expressed by the vector comprises a histidine tag, an orally-taken immunologic adjuvant cholera toxin B subunit (CTB) and six B cellular antigen epitopes Bp of refractory hepatitis c virus genetype 1. To facilitate high-efficiency expression of the vaccine in tobacco, the 6His-CTBt-Bt gene optimized by an artificially synthesized codon is cloned to a high-efficiency plant virus expression vector potato X virus vector PVX201 to obtain the recombinant expression vector PVX-6His-CTBt-Bt of the multi-epitope vaccine 6His-CTBt-Bt of the hepatitis c virus; the expression vector is inoculated to the tobacco; the tobacco is regarded as a reactor to produce the anti-hepatitis c virus vaccine which aims to the refractory hepatitis c virus, is easy to purify, and can achieve a good orally-taken immune effect and resist against virus infection in vitro.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Efficient cell culture system for hepatitis c virus genotype 2b
InactiveUS20110294194A1High mutation rateReduce inhibitionSsRNA viruses positive-senseSugar derivativesSequence analysisHCV Genotyping
The present inventors developed hepatitis C virus 2b / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and the complete NS2 were replaced by the corresponding genes of the genotype 2b reference strain J8. Sequence analysis of recovered 2b / 2a recombinants from 2 transfection experiments revealed that 2b / 2a was genetically stable. Conclusion: The developed 2b / 2a viruses provide a robust in vitro tool for research in HCV genotype 2b, including vaccine studies and functional analyses.
Owner:HVIDOVRE HOSPITAL
Hepatitis C virus genotyping method based on loop-mediated isothermal amplification
InactiveCN109097494AIncreased sensitivityStrong specificityMicrobiological testing/measurementBiotechnologyHepatitis C virus genotype
The invention relates to a hepatitis C virus genotyping method based on loop-mediated isothermal amplification, and relates to the field of molecular biotechnology. The hepatitis C virus genotyping method is characterized in that different subtypes of LAMP primers are designed based on the LAMP method specific to the specific gene sequences of the subtypes in the 5'UTR-Core region of the hepatitisC virus, including two outer primers, two inner primers and a loop primer, and an NEB reagent is adopted for detection at 65 DEG C for 60 min. The method has high specificity, high sensitivity and low cost, can achieve high-throughput automatic detection and contamination risks of specimens. Low equipment requirements can help promote the clinical application of the LAMP method. The method has good clinical application prospects. At the same time, the development of corresponding reagents is conducive to further promoting the clinical application of the method.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
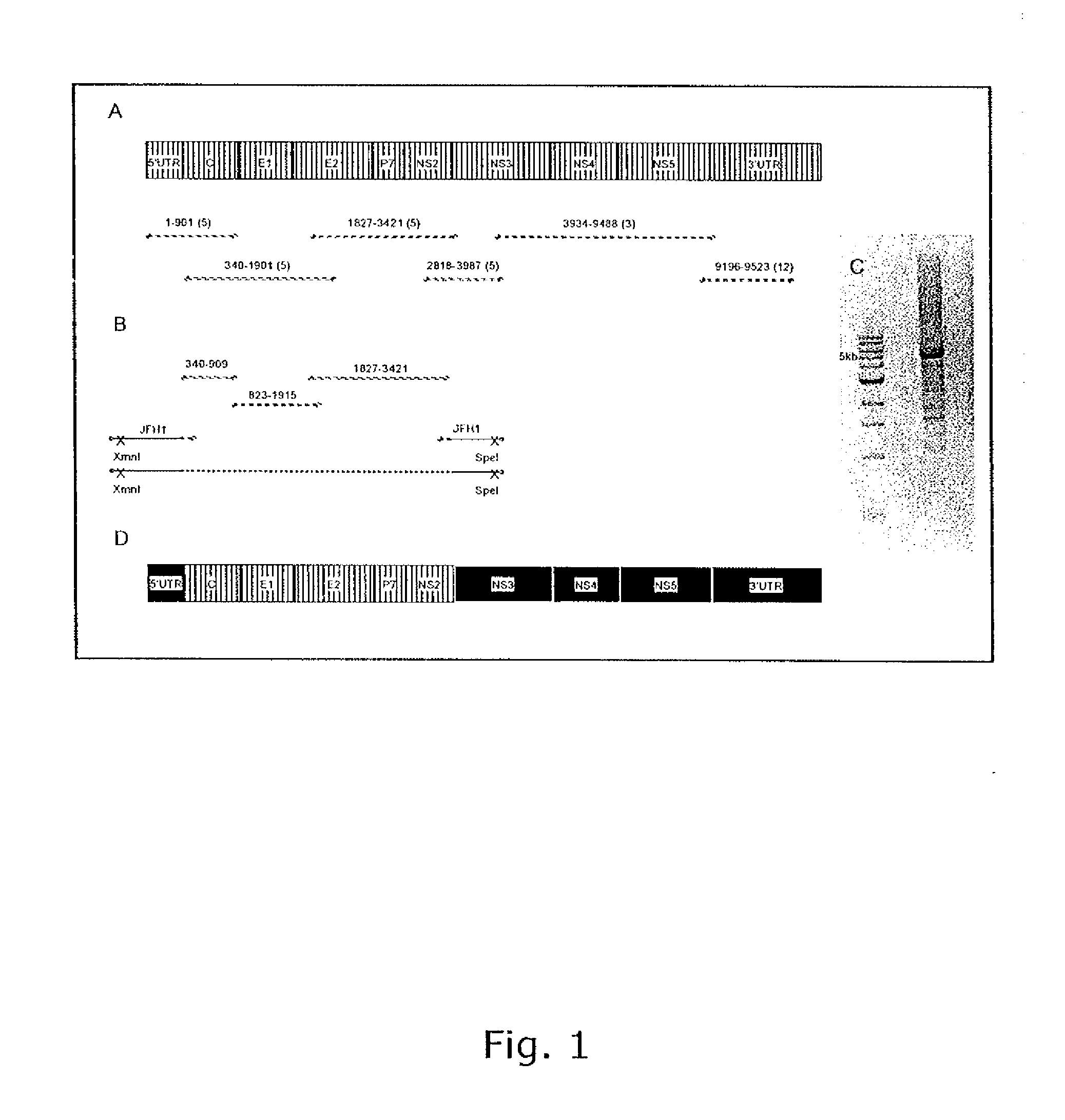
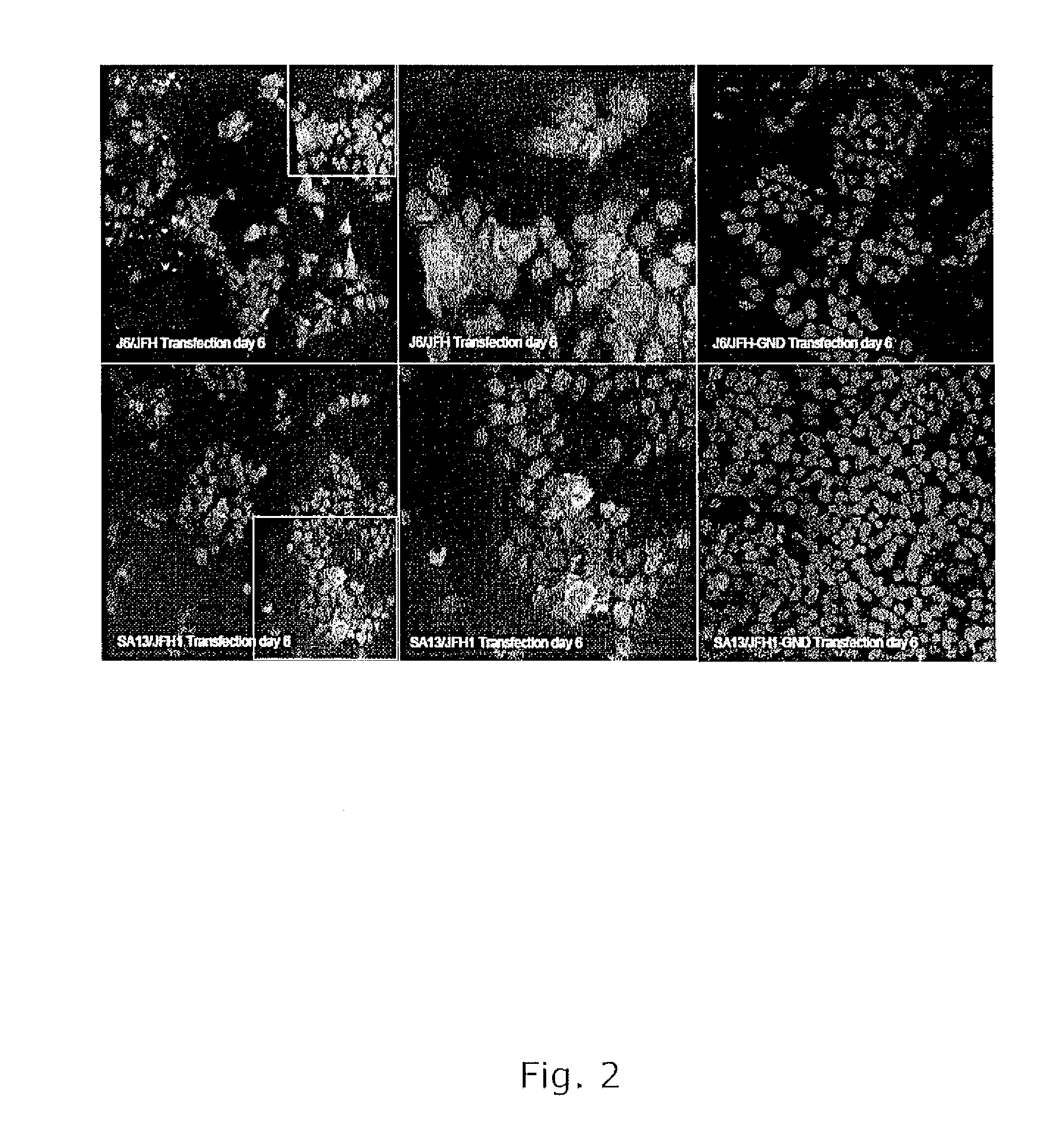
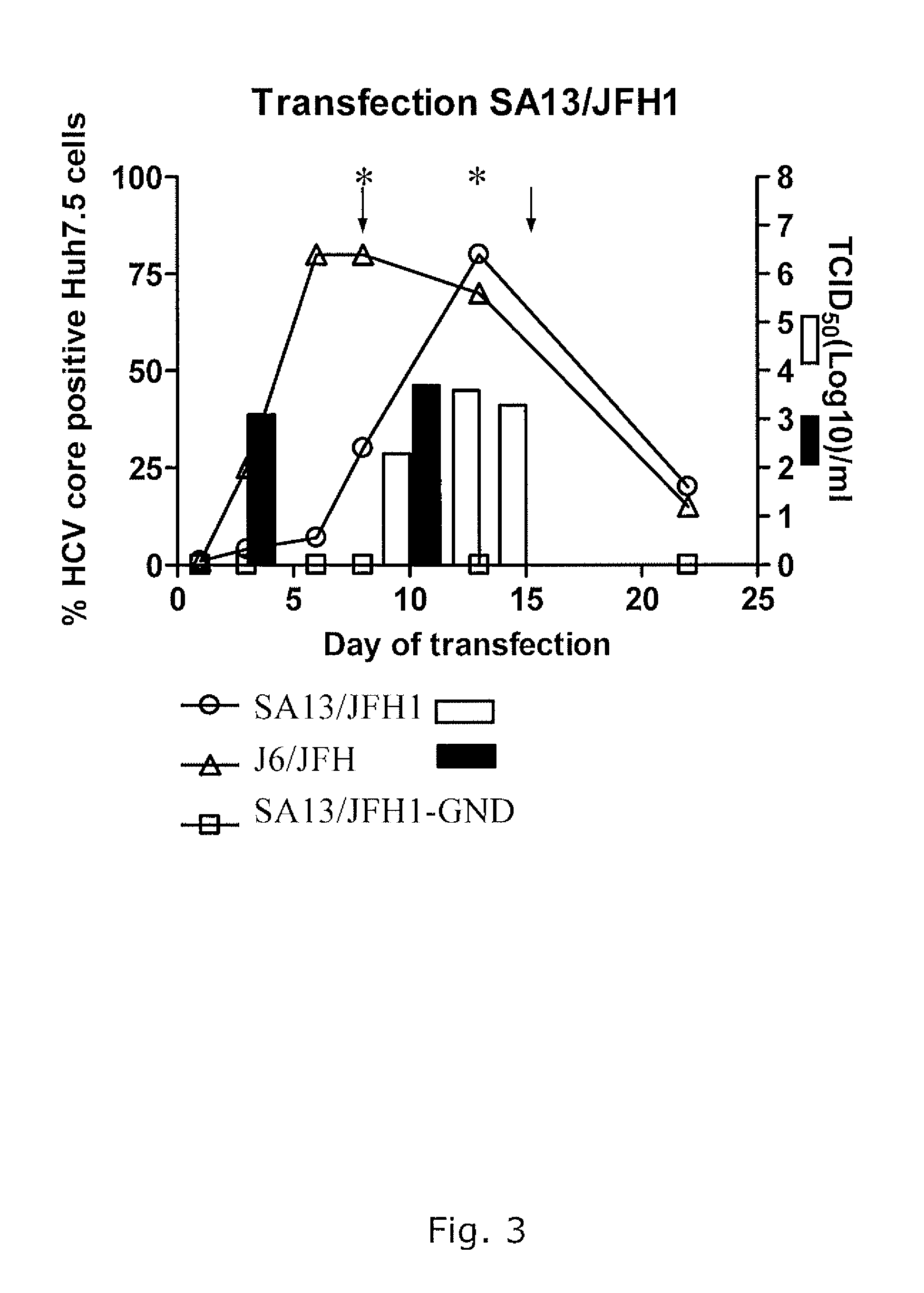
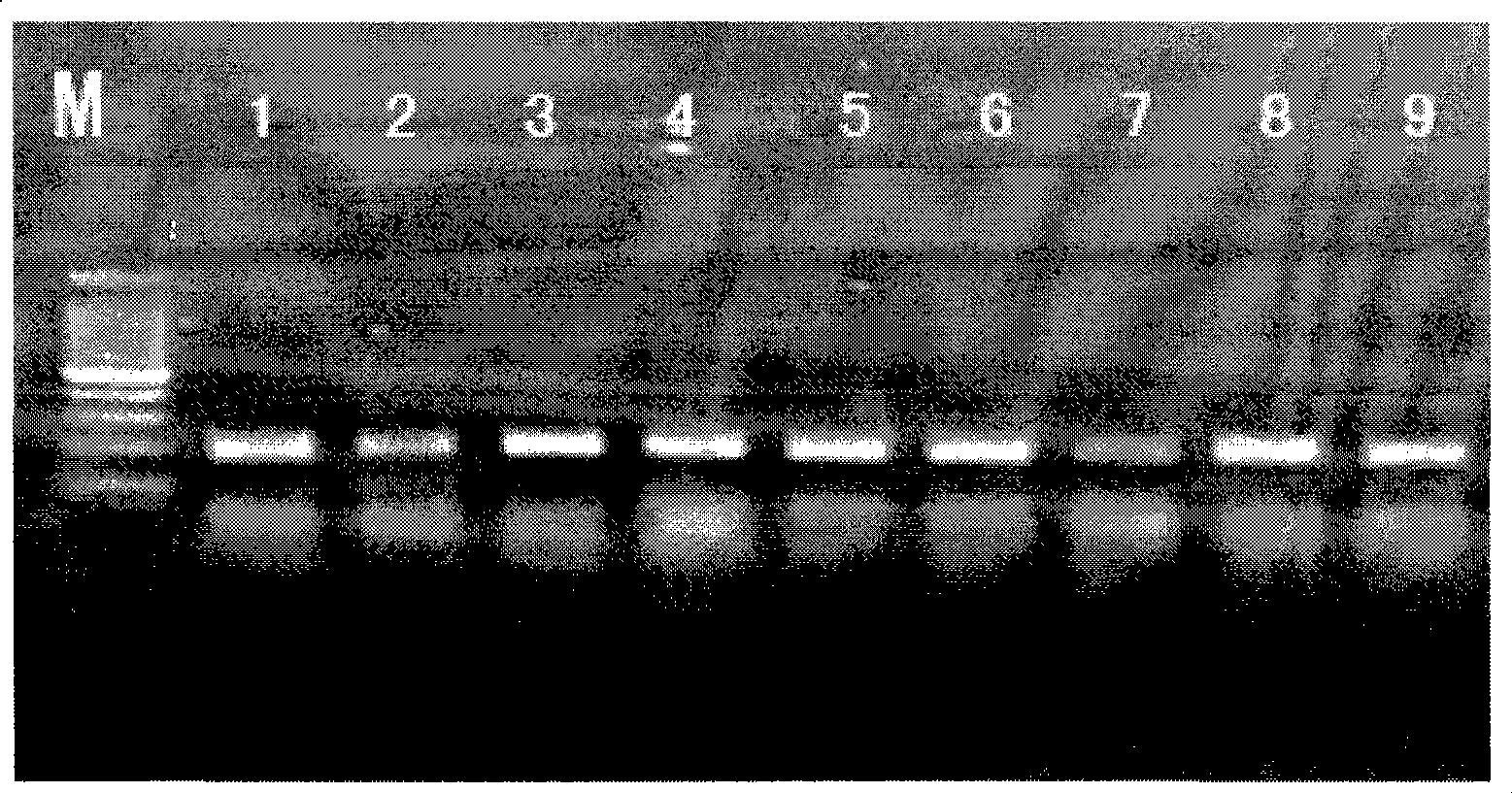
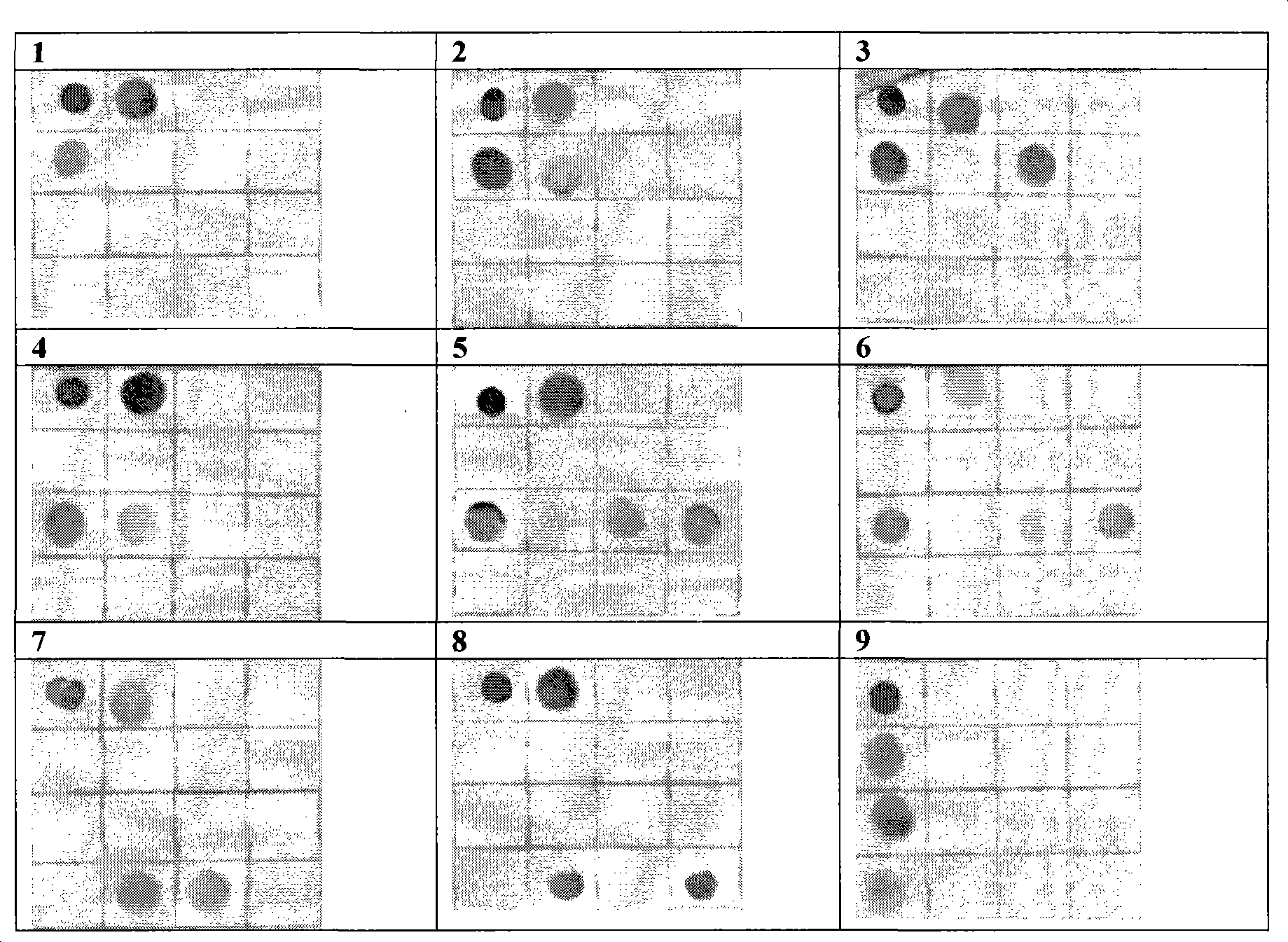
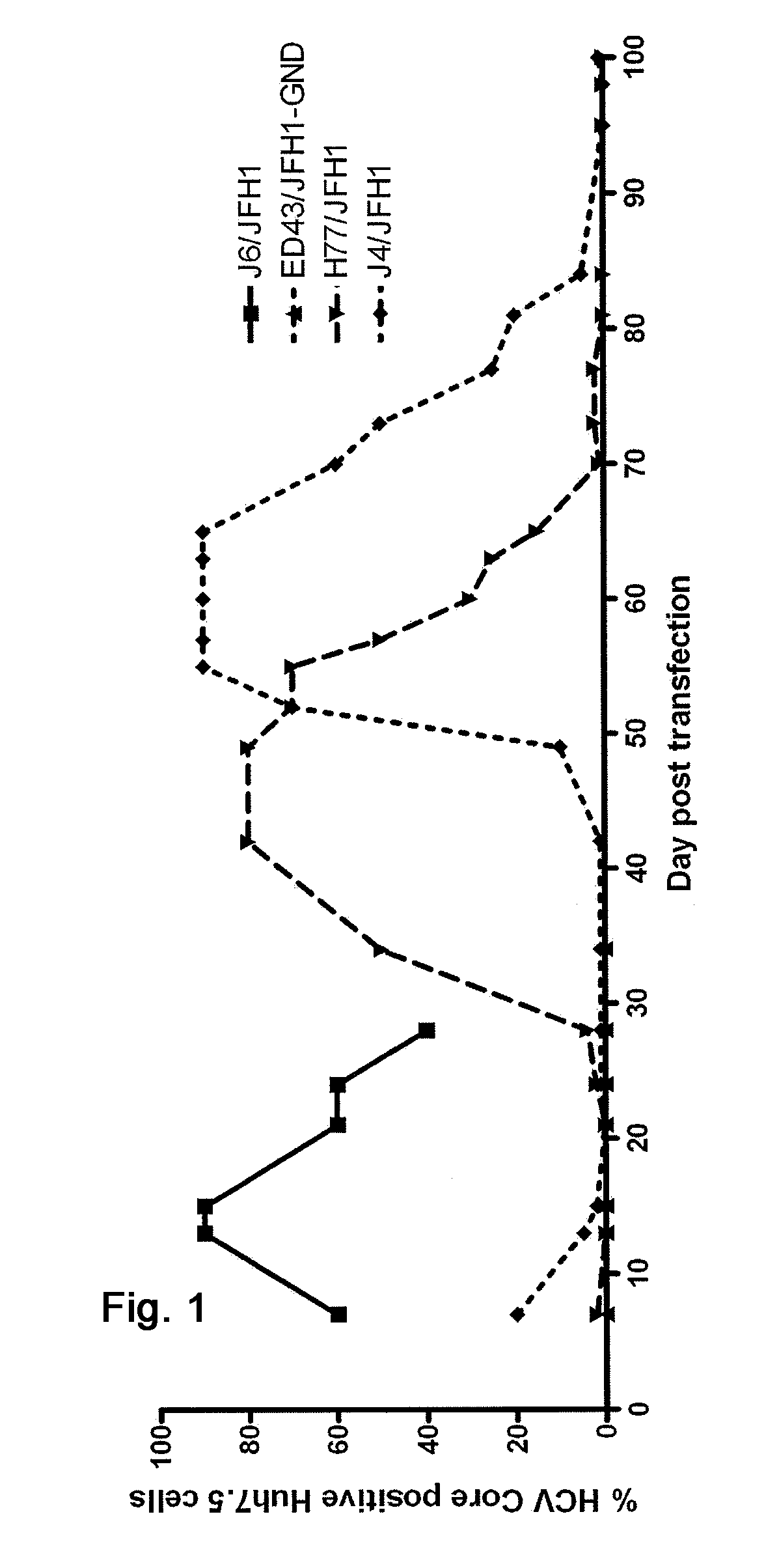
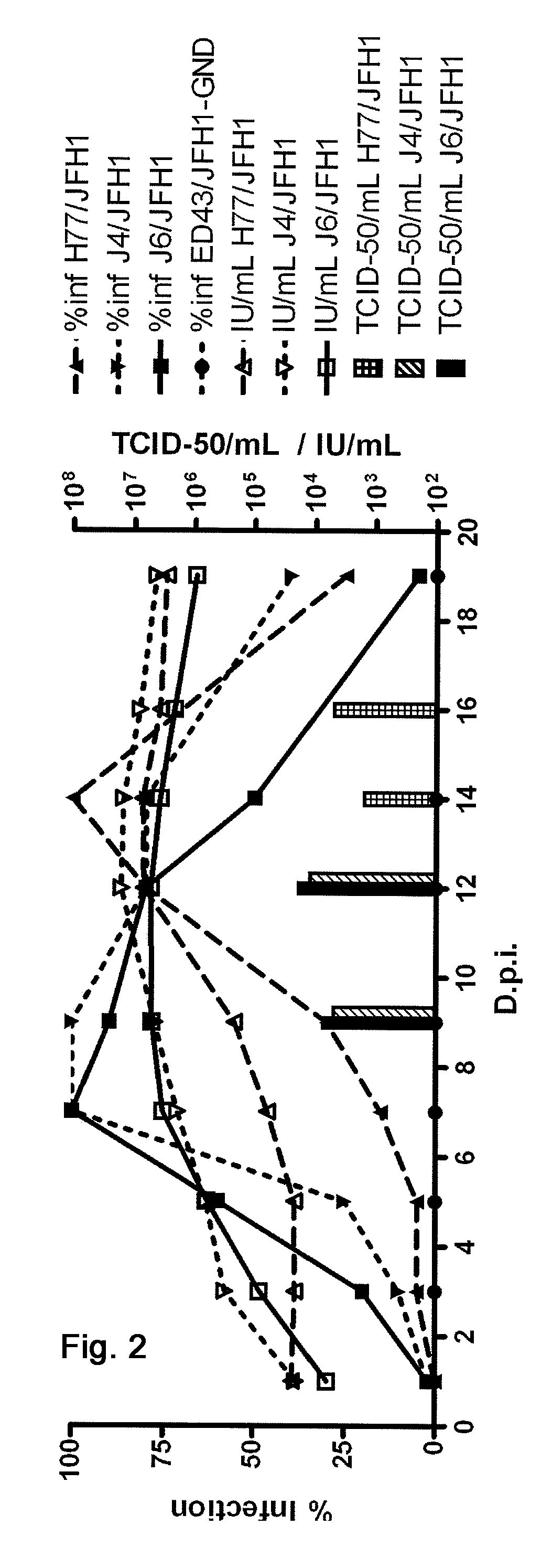
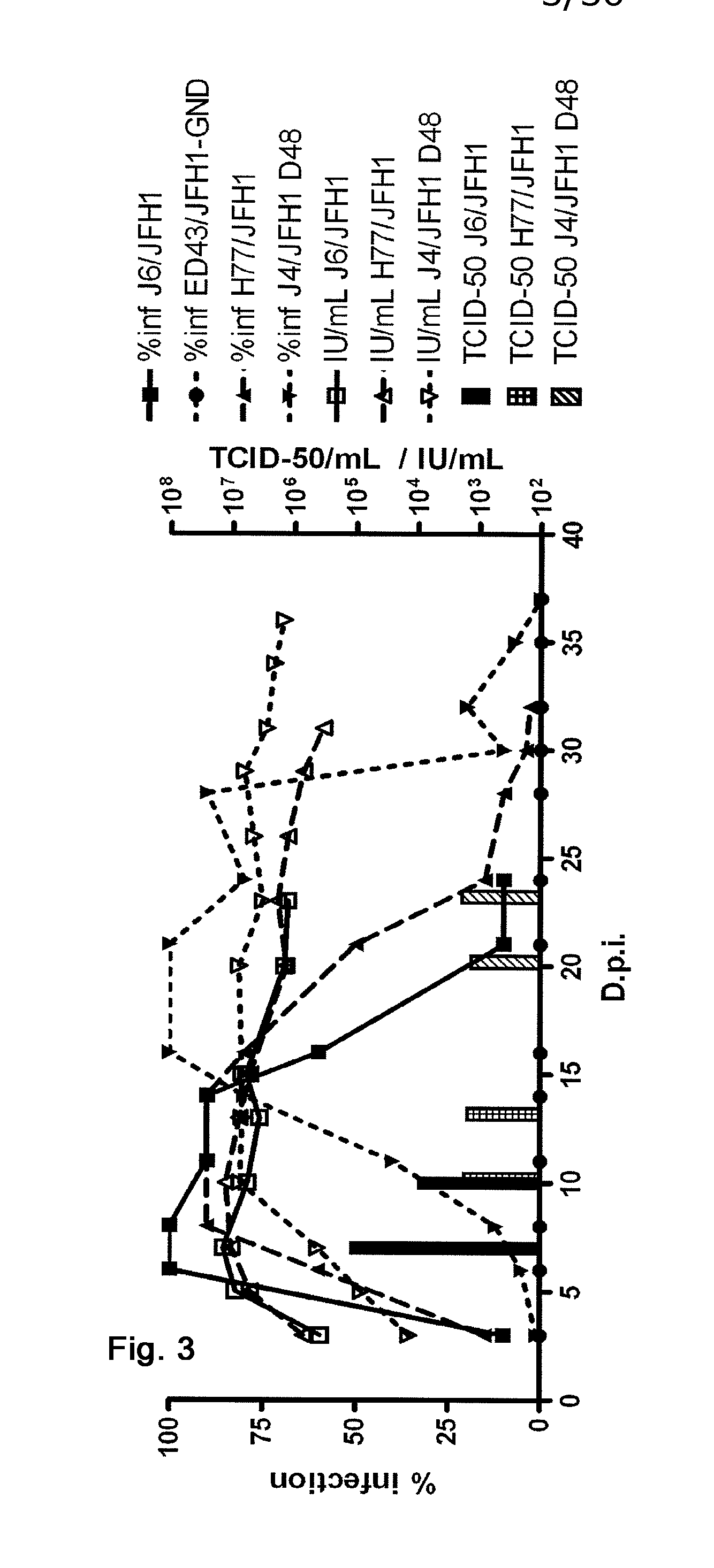
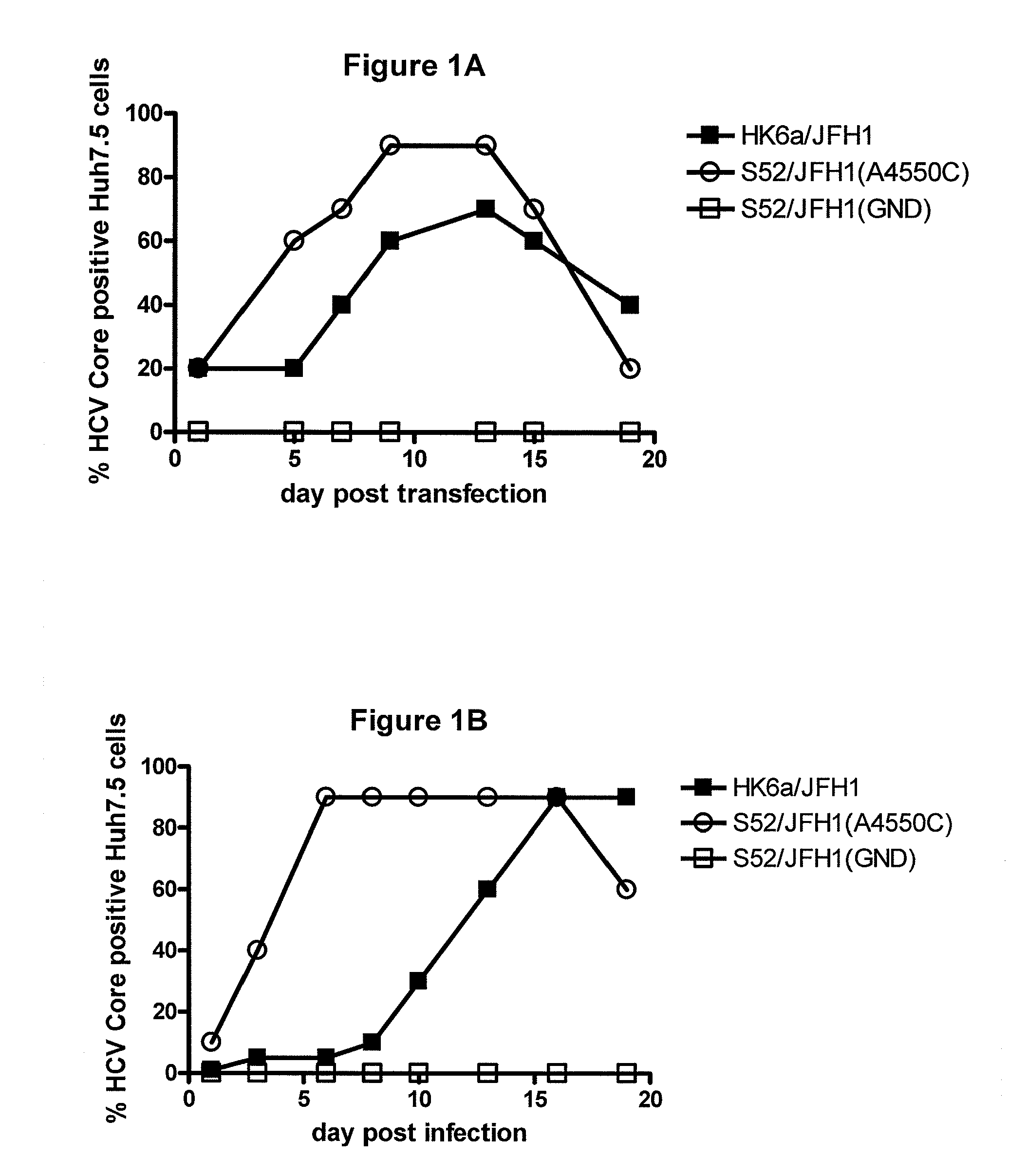
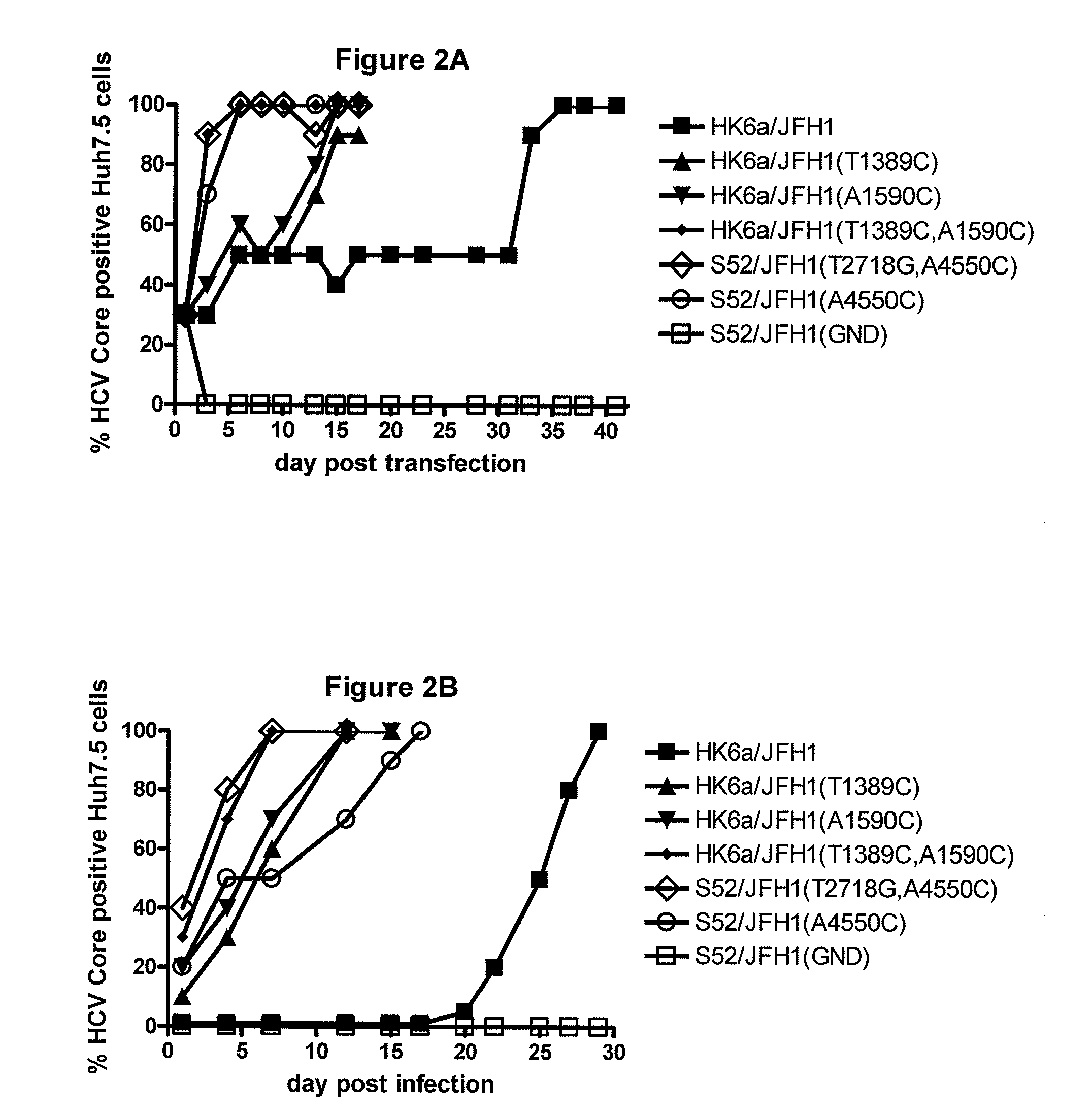
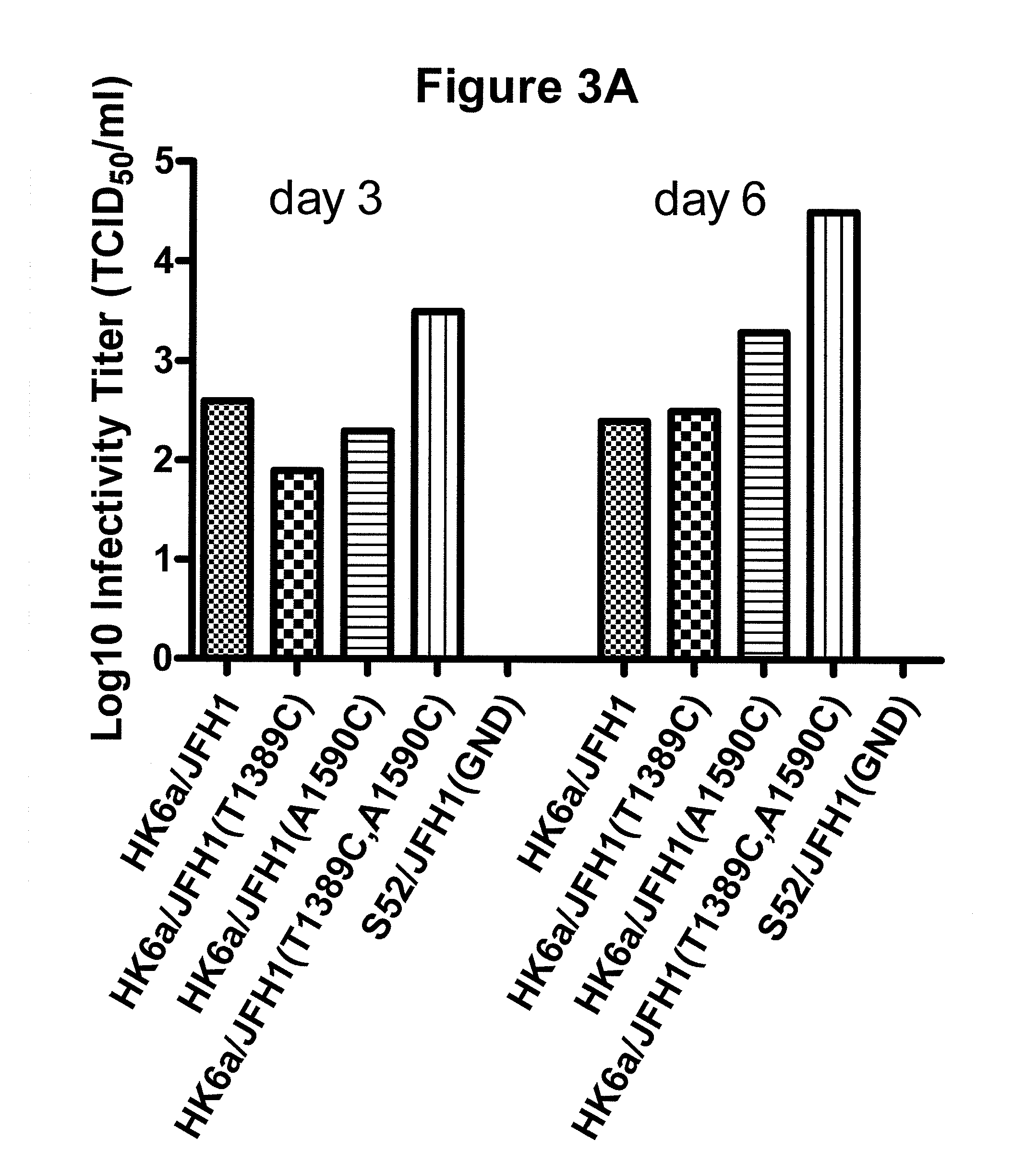
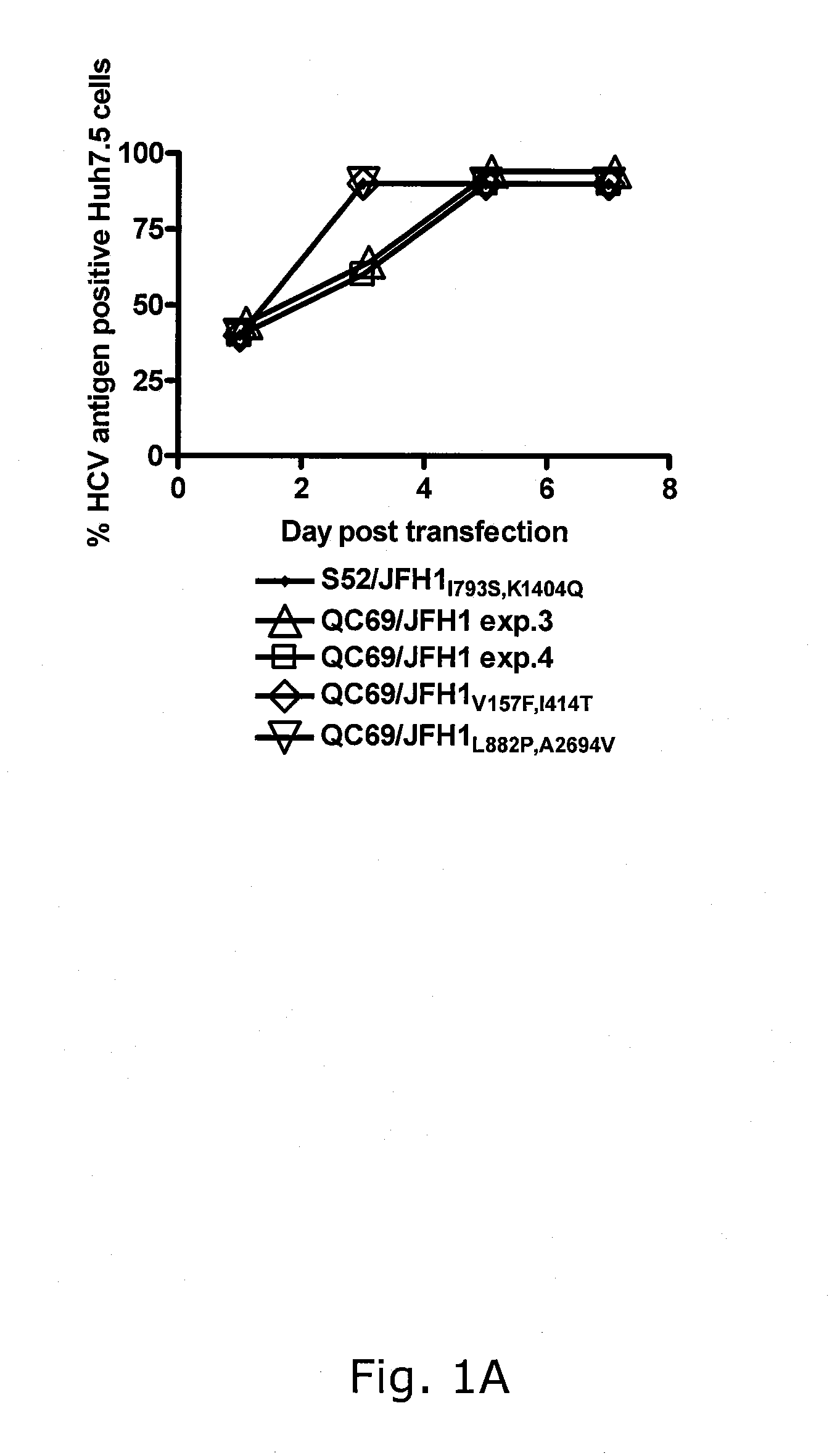
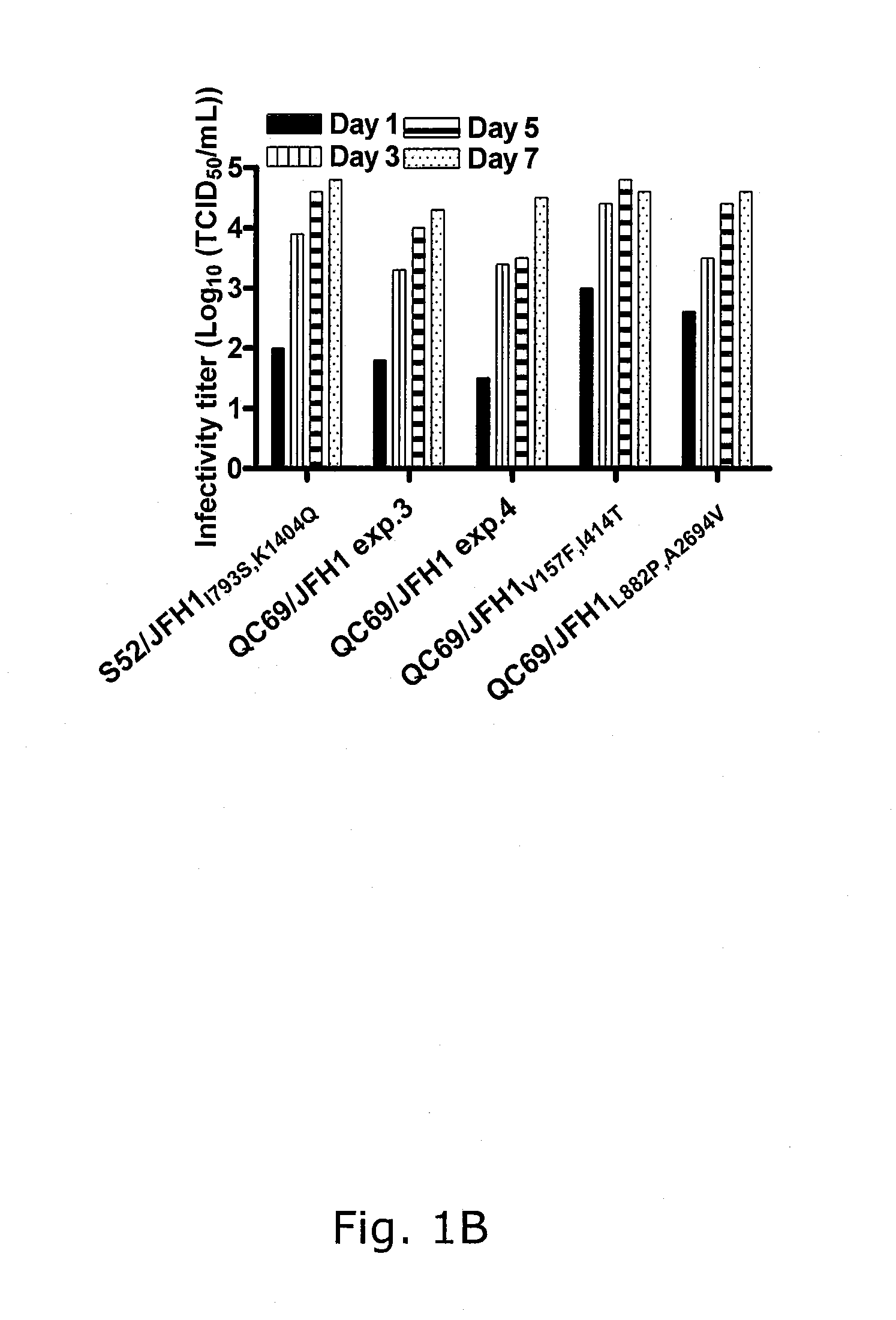
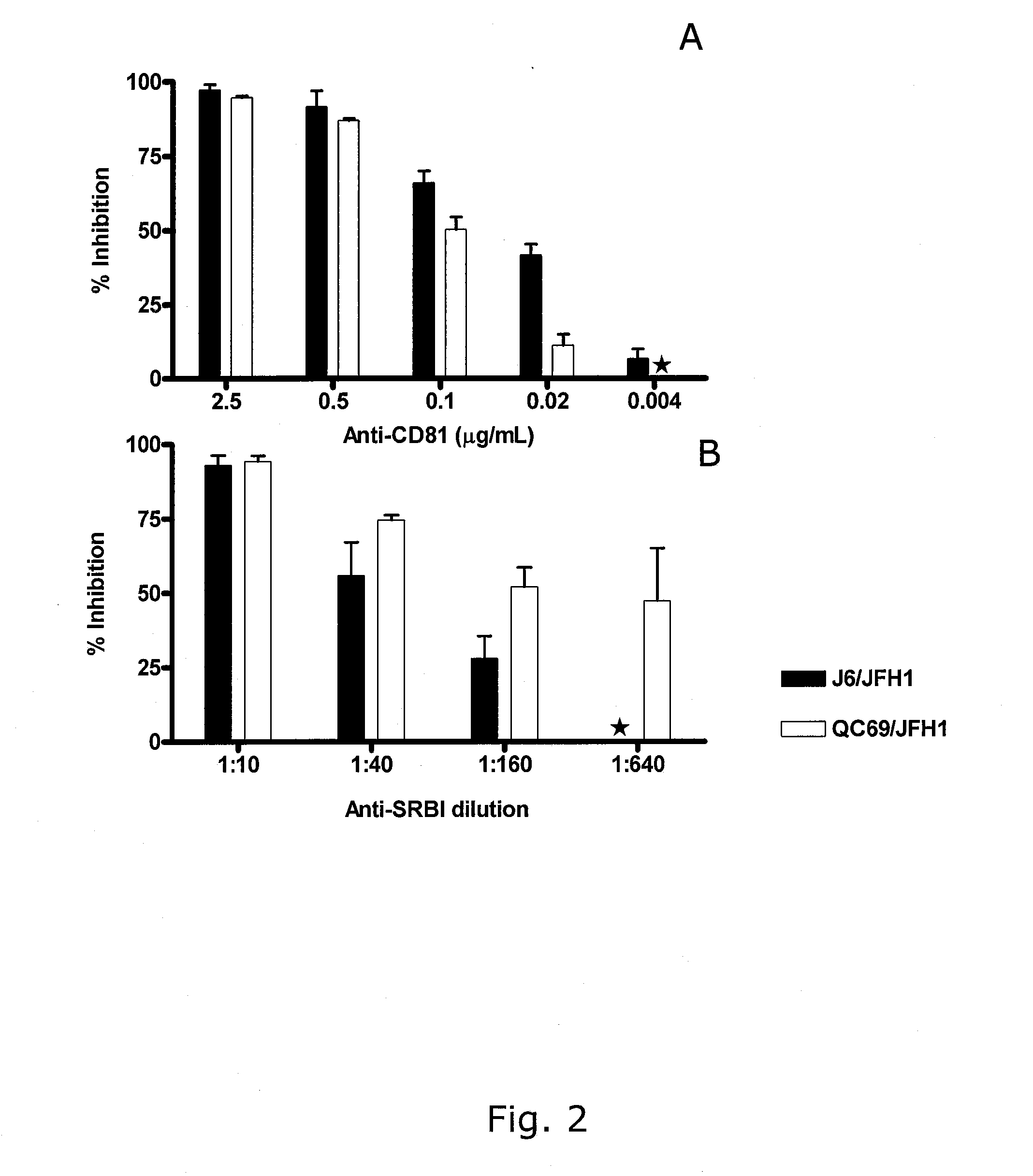
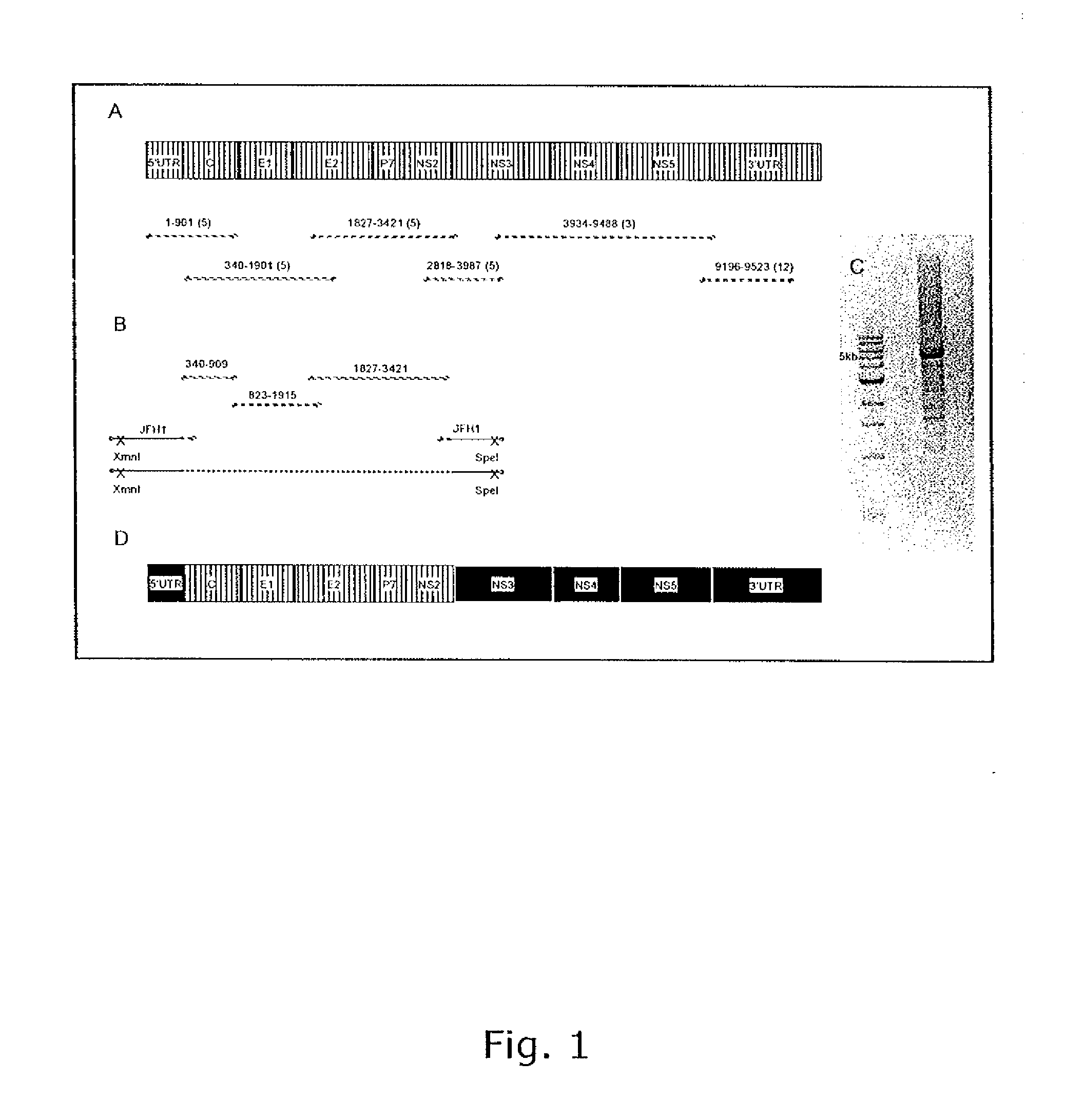
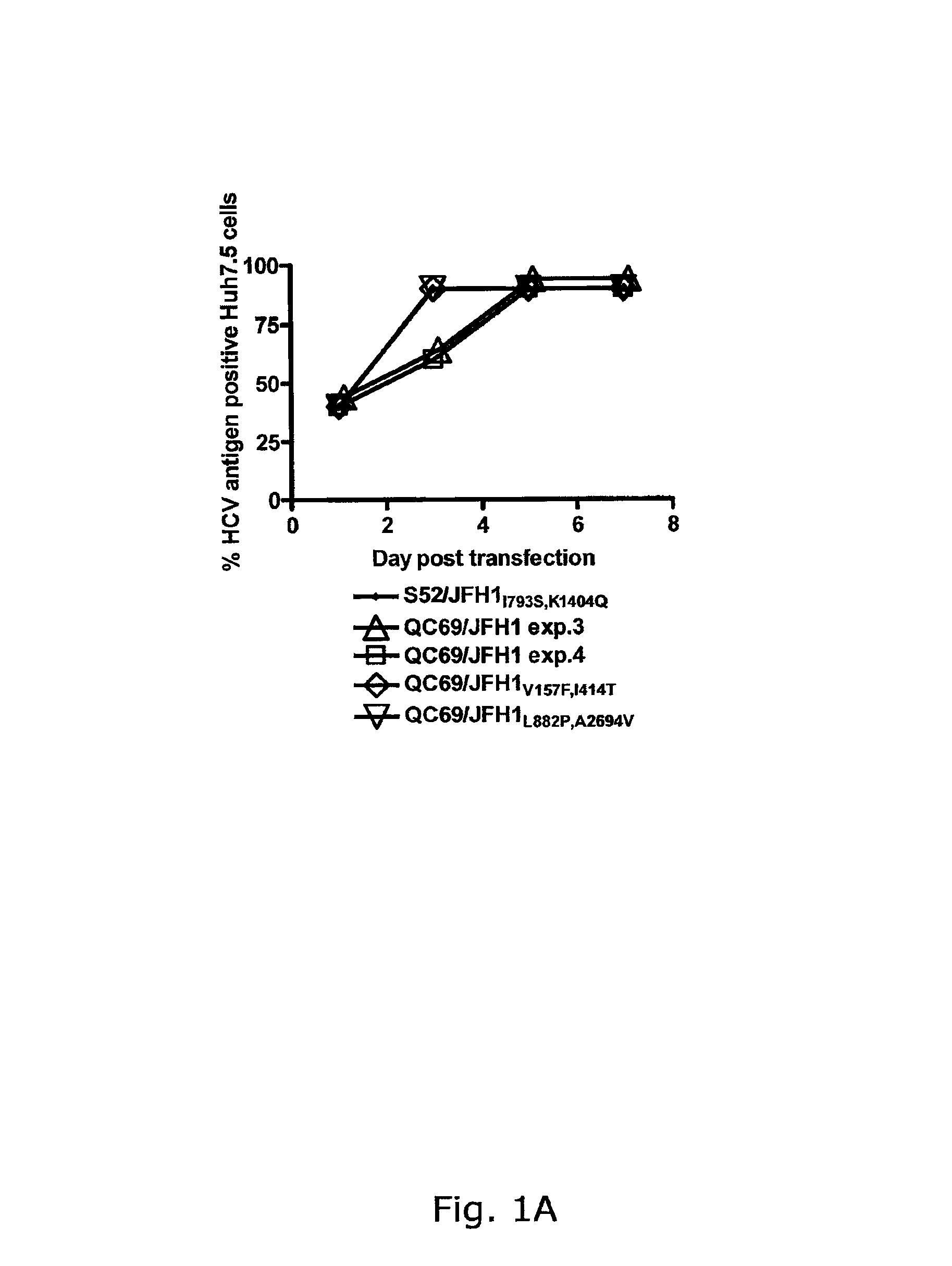
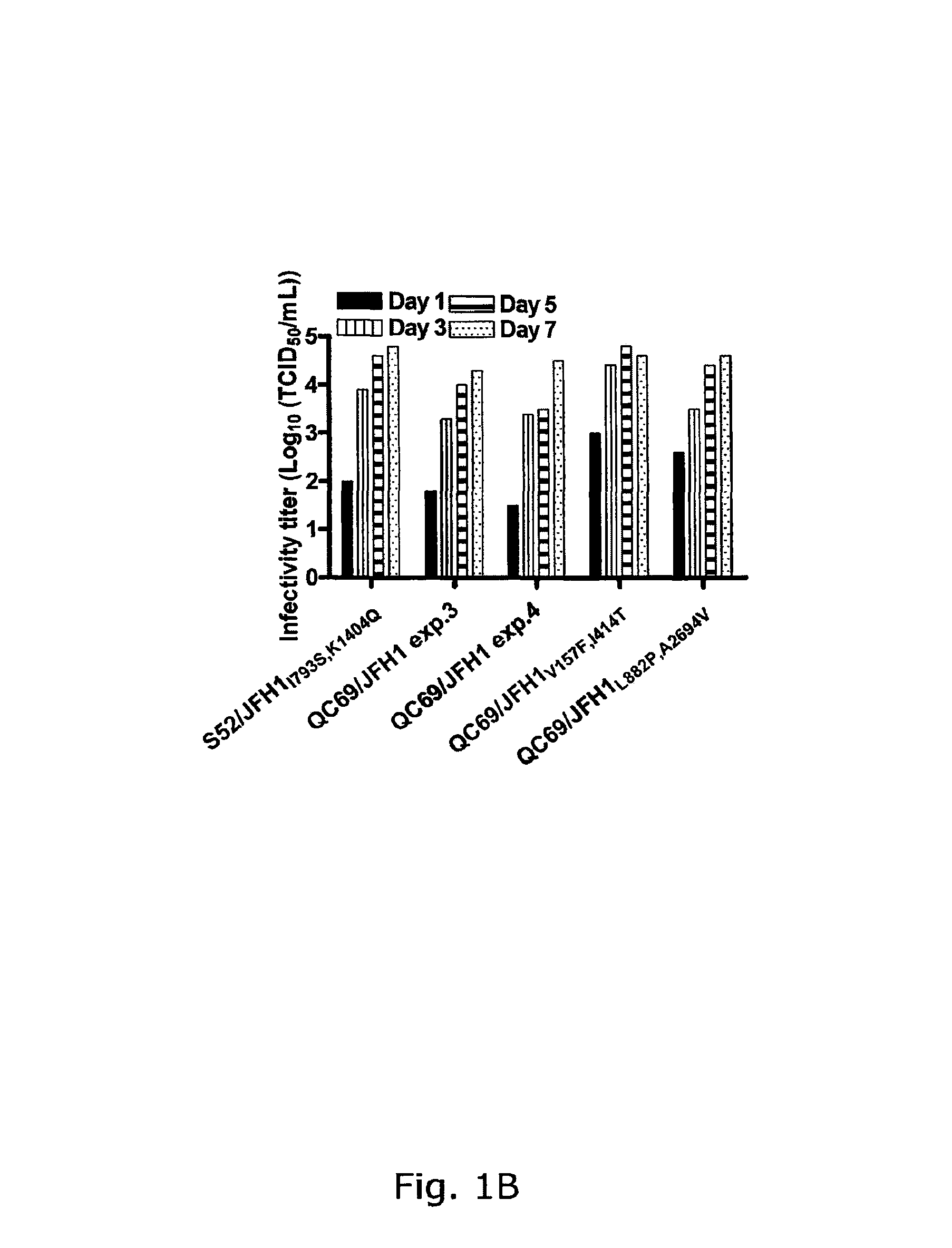
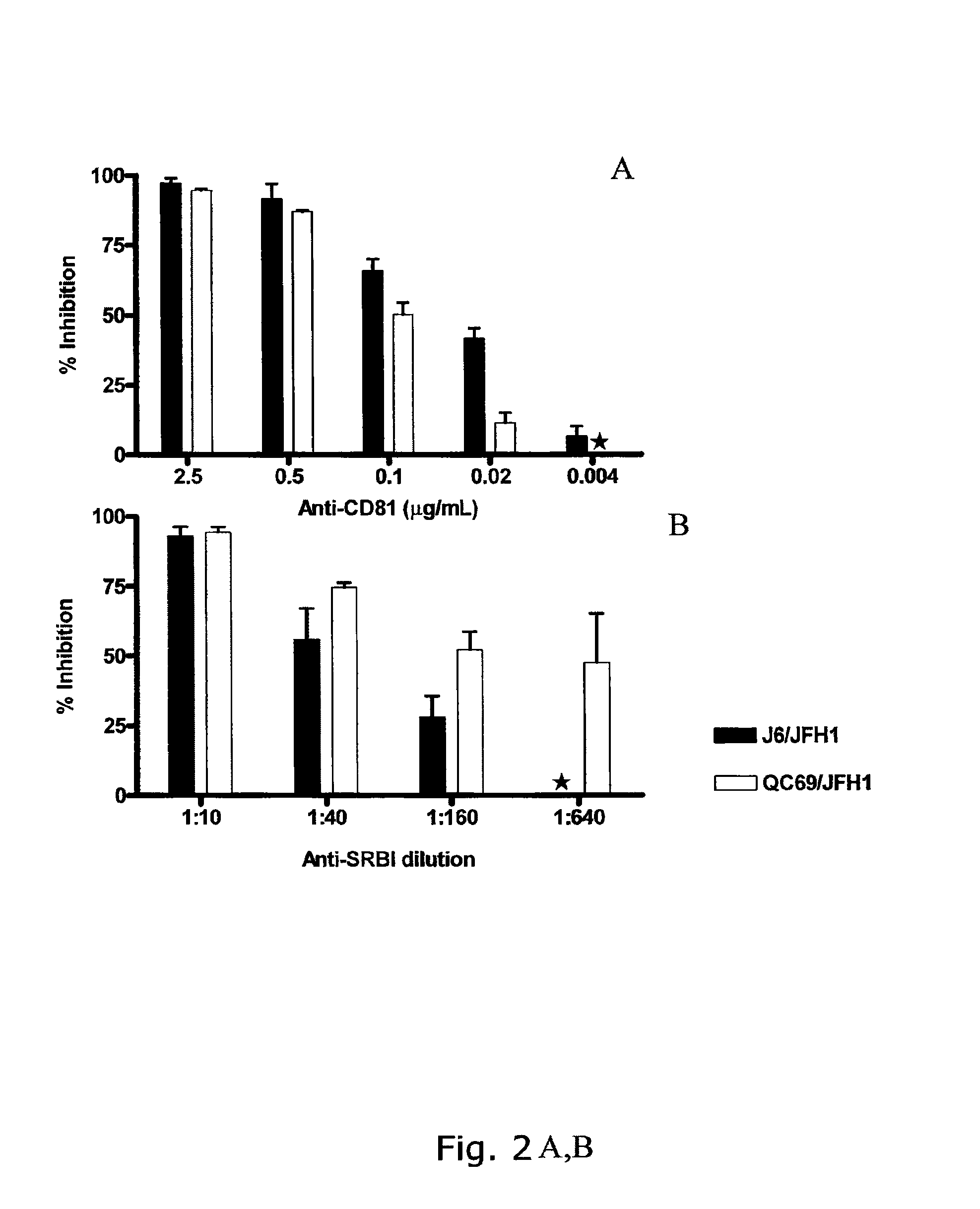
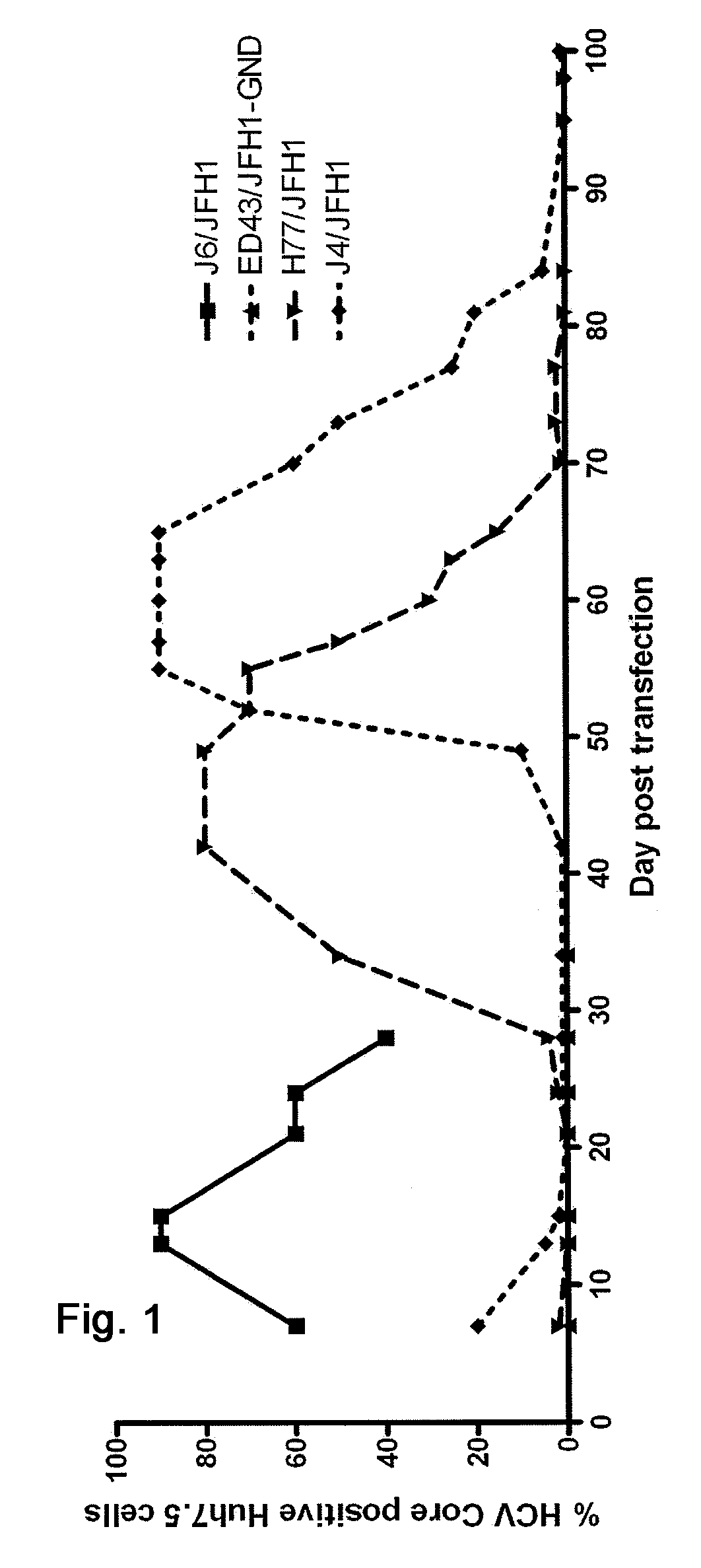
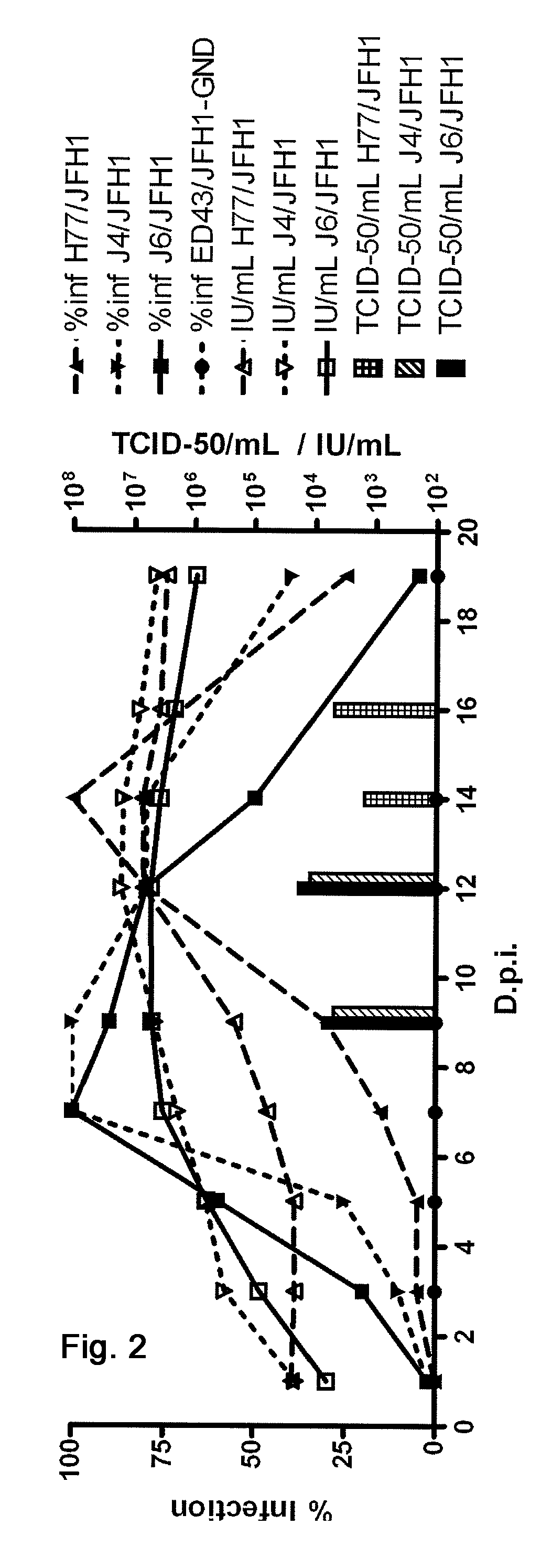
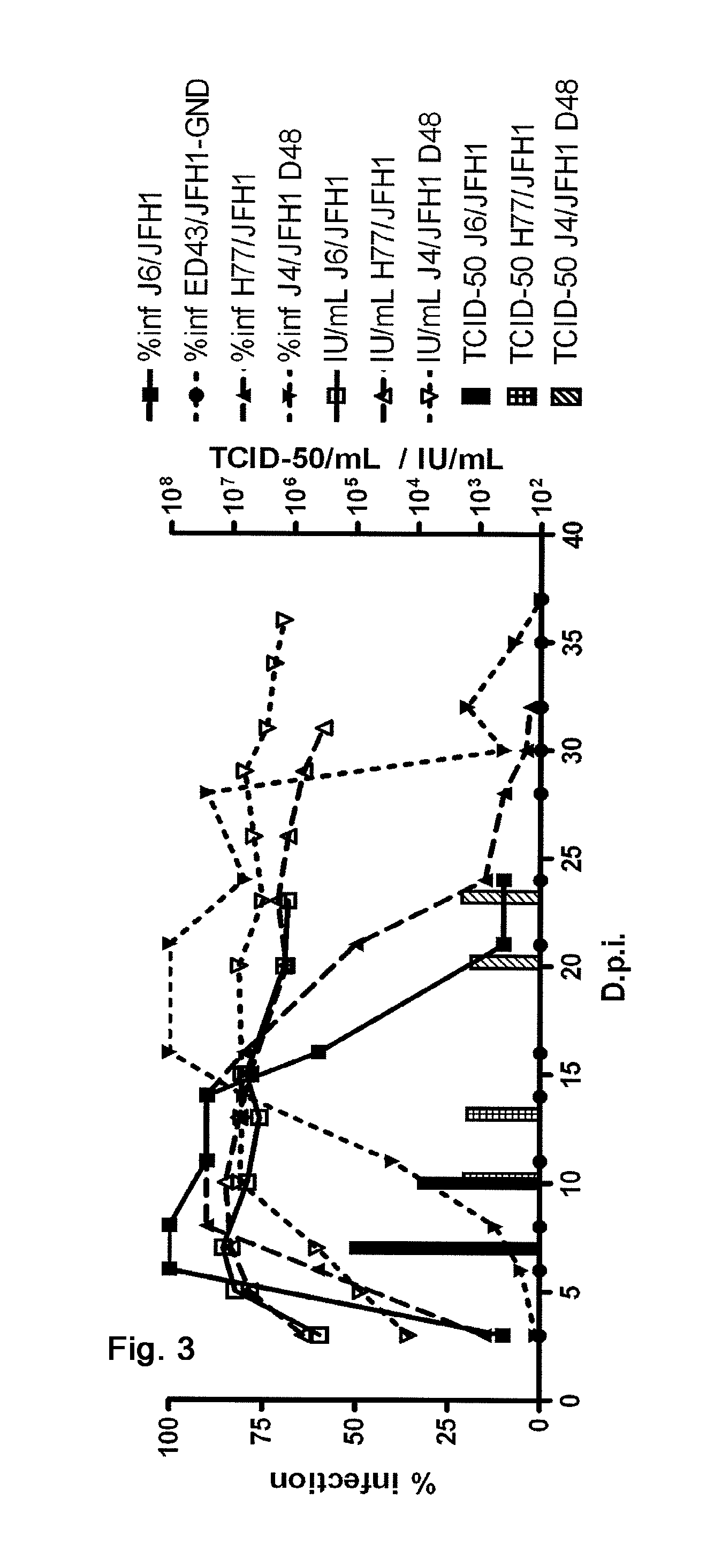
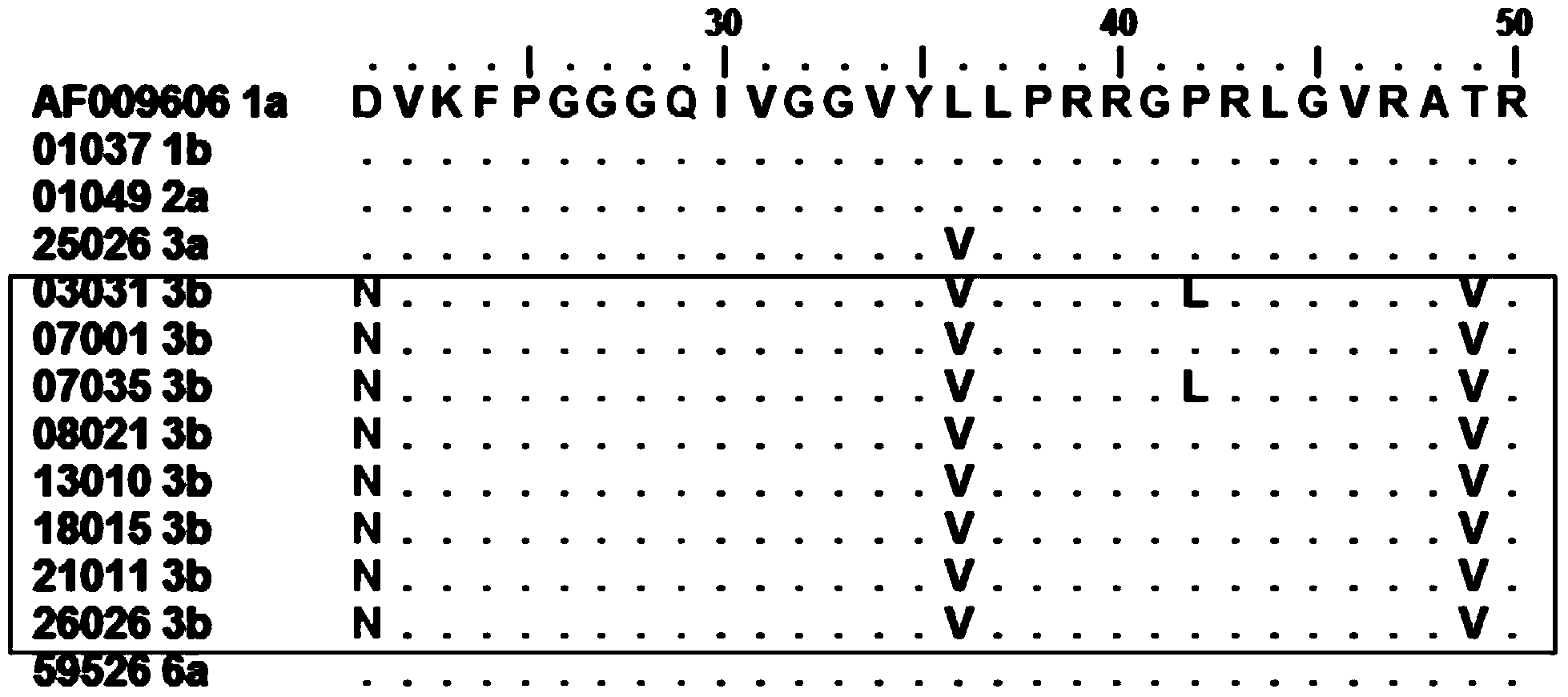
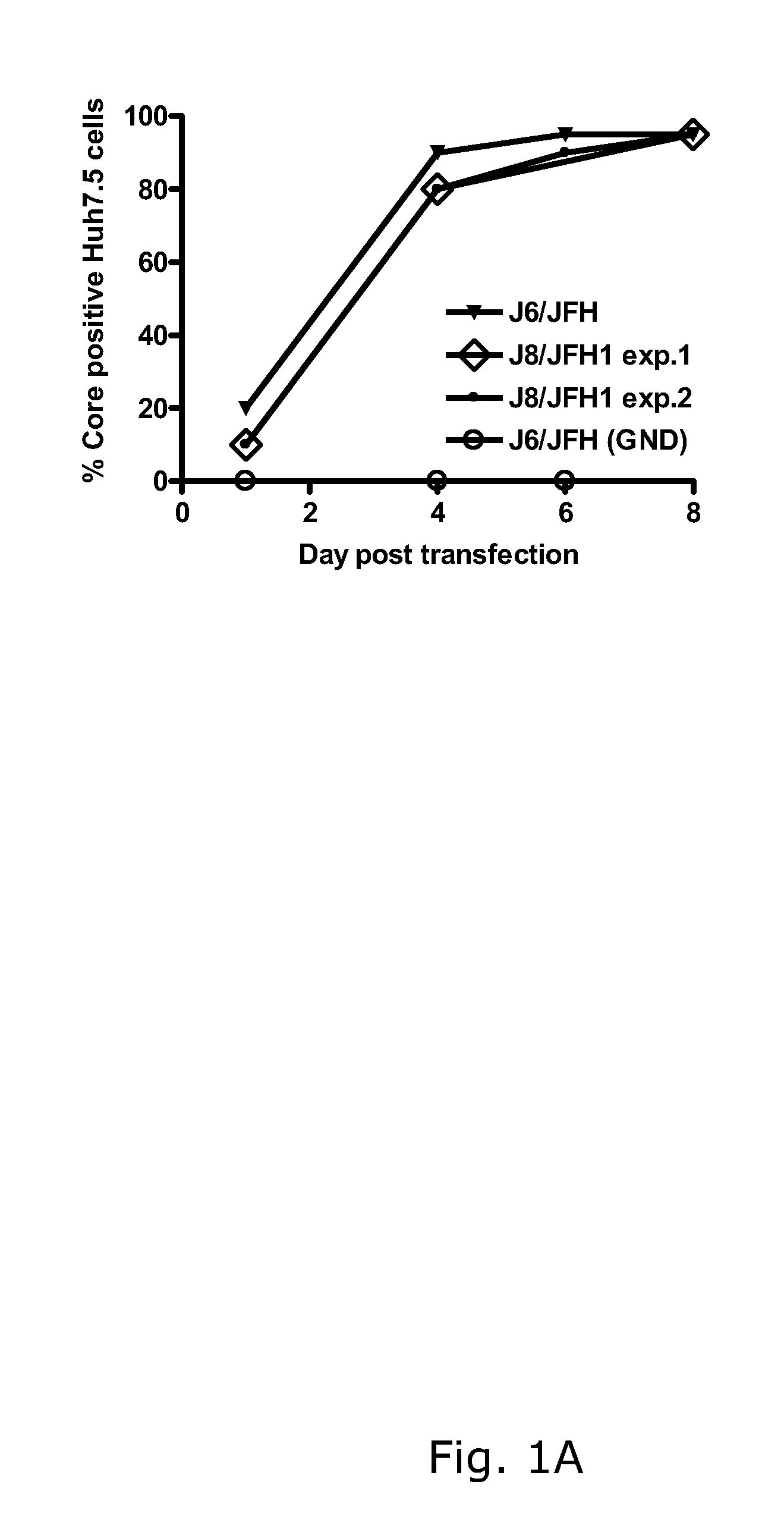
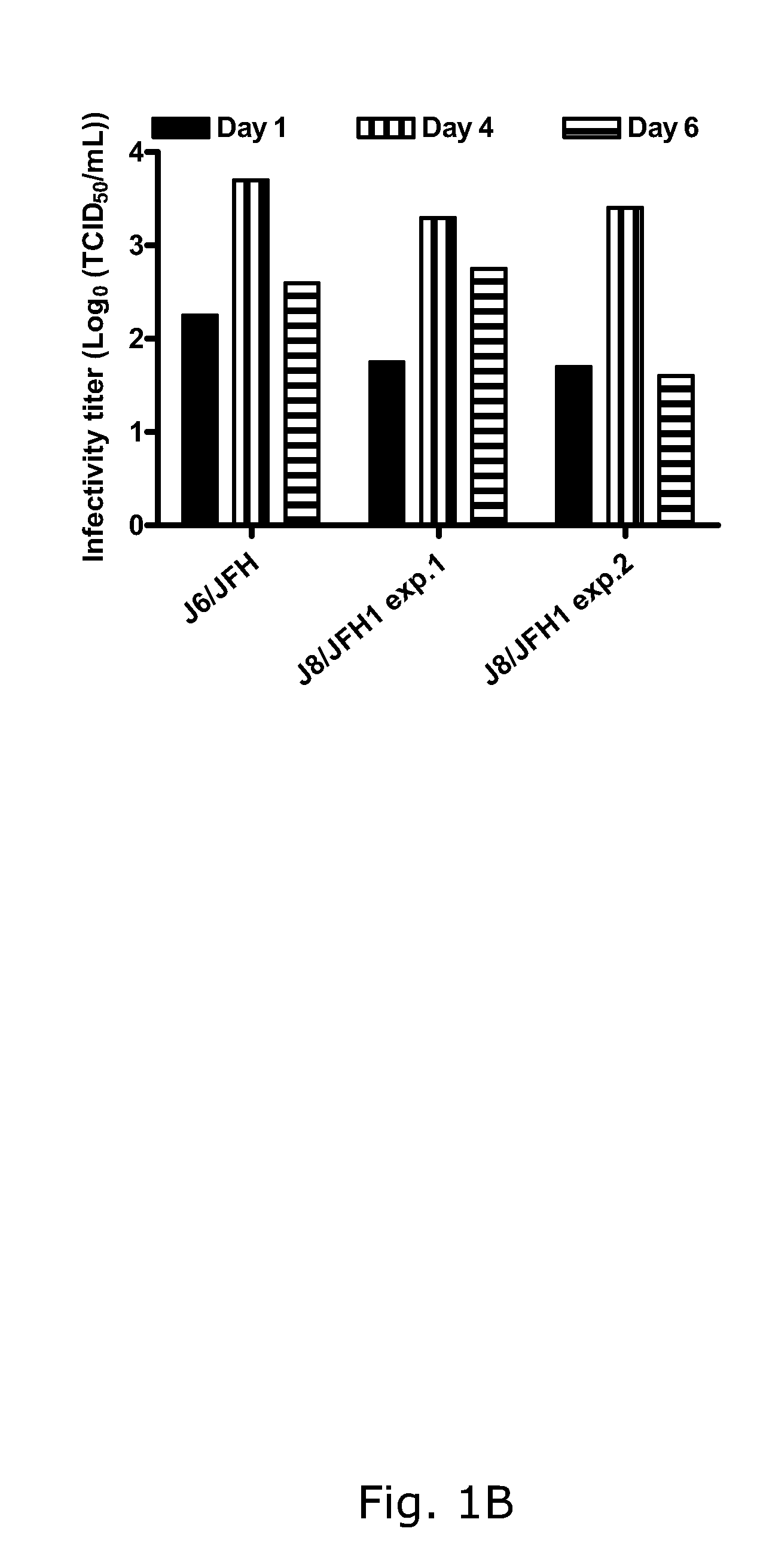
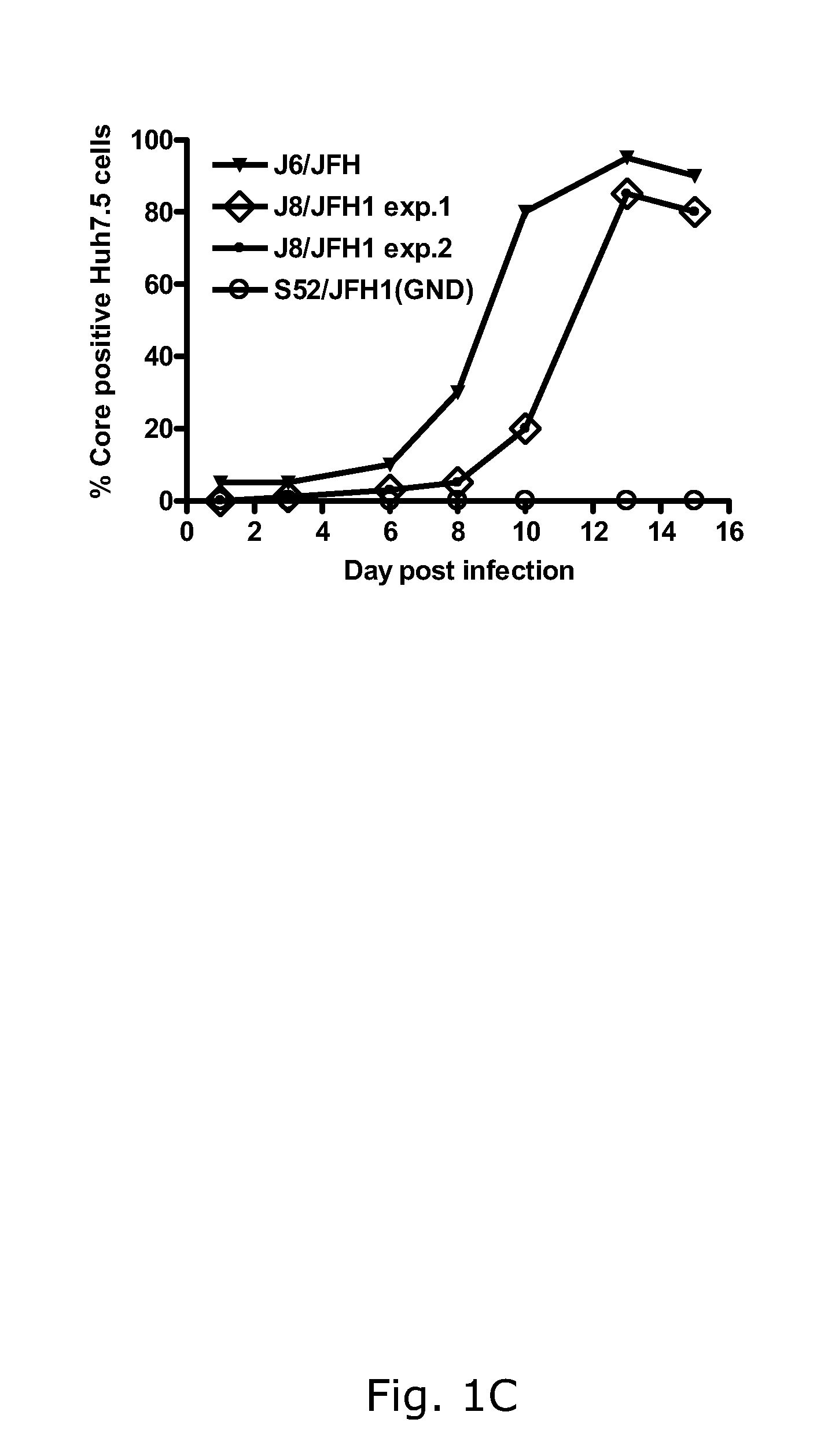
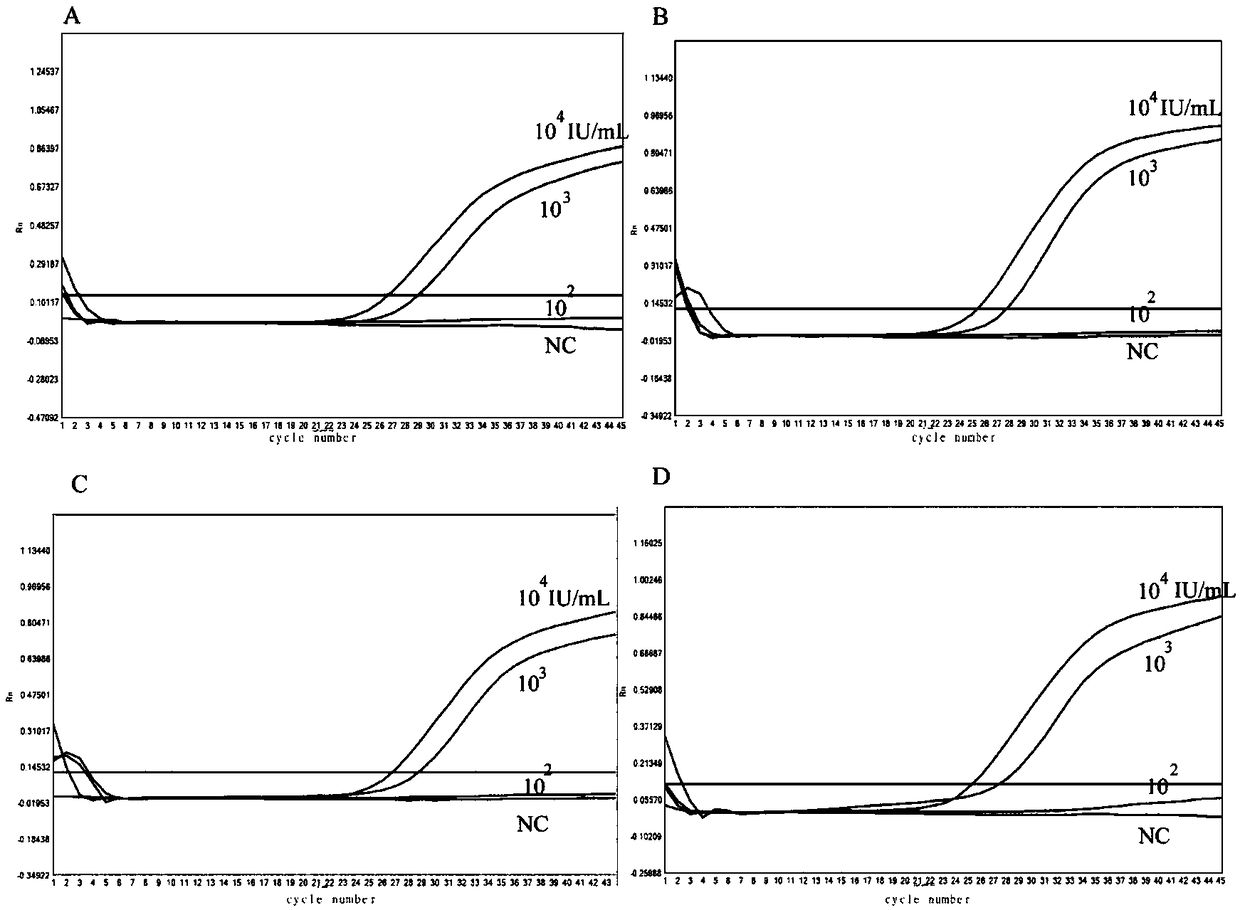
Nucleic acid construct including nucleic acid derived from genotype 3[alpha] HCV genome
InactiveCN104126008ASsRNA viruses positive-senseMicrobiological testing/measurementNucleotideHepatitis C virus genotype
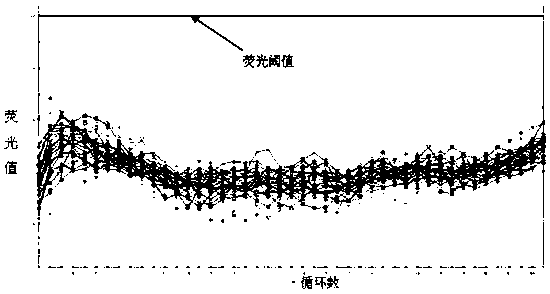
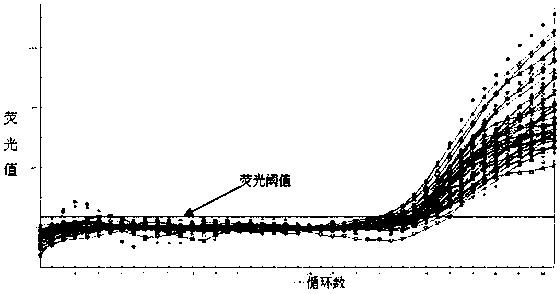
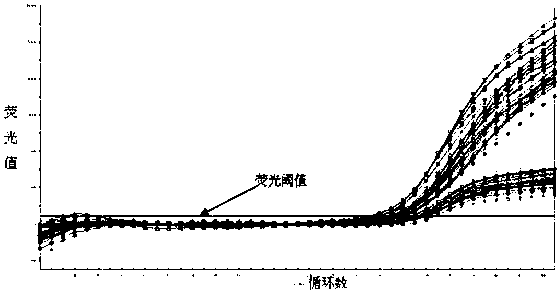
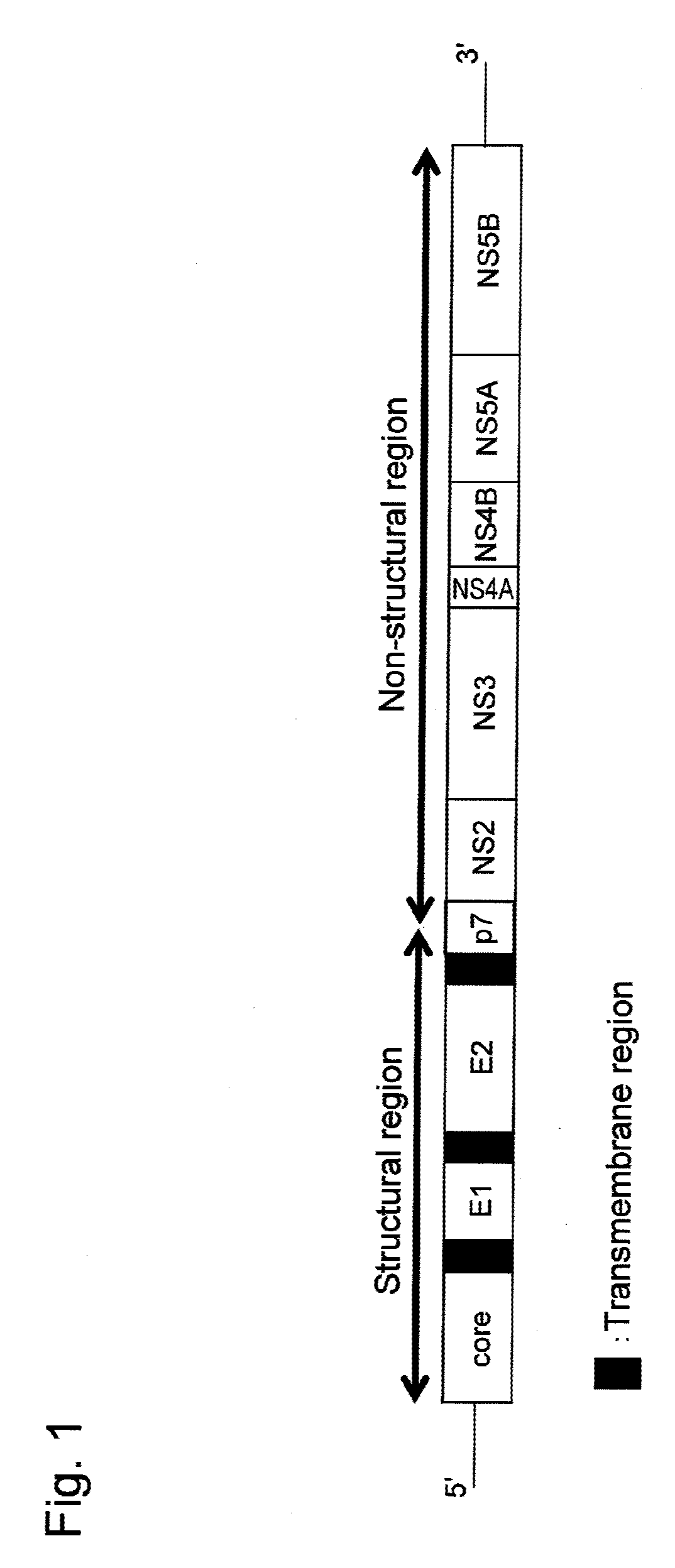
This invention provides a nucleic acid comprising, in the following order, a 5' untranslated region comprising a particular nucleotide sequence of the genome of hepatitis C virus genotype 3a; a nucleotide sequence encoding a particular amino acid sequence of an NS3 protein, a nucleotide sequence encoding a particular amino acid sequence of an NS4A protein, a nucleotide sequence encoding a particular amino acid sequence of an NS4B protein, a nucleotide sequence encoding a particular amino acid sequence of an NS5A protein, a nucleotide sequence encoding a particular amino acid sequence of an NS5B protein of the hepatitis C virus genotype 3a; and a 3' untranslated region comprising a particular nucleotide sequence of a genome of hepatitis C virus genotype 3a.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES +2
New treatments of hepatitis c virus infection
The invention concerns the use of cyclophilin inhibitors in the treatment of Hepatitis C virus genotype 2 or 3 infection.
Owner:NOVARTIS AG +1
HCV (Hepatitis c virus) genotype detection kit
ActiveCN103710464BStrong specificityHigh purityMicrobiological testing/measurementMicroorganism based processesMagnetic beadFluorescence
The invention relates to a HCV (hepatitis c virus) genotype detection kit. The kit extracts a sample nucleic acid by using a magnetic bead method; by using a real-time fluorescence quantitative PCR (polymerase chain reaction) technology, and taking a highly conserved area of a HCV genome as an amplification target, a specific primer and a TaqMan probe are designed, then HCV genes are subjected to rapid and accurate genotyping detection on a real-time fluorescence PCR instrument by PCR amplification, and an internal standard is added in a system, so that false negative results can be effectively prevented.
Owner:SANSURE BIOTECH INC
Amplification primer, sequencing primer, kit and method for detection of hepatitis C virus genotyping
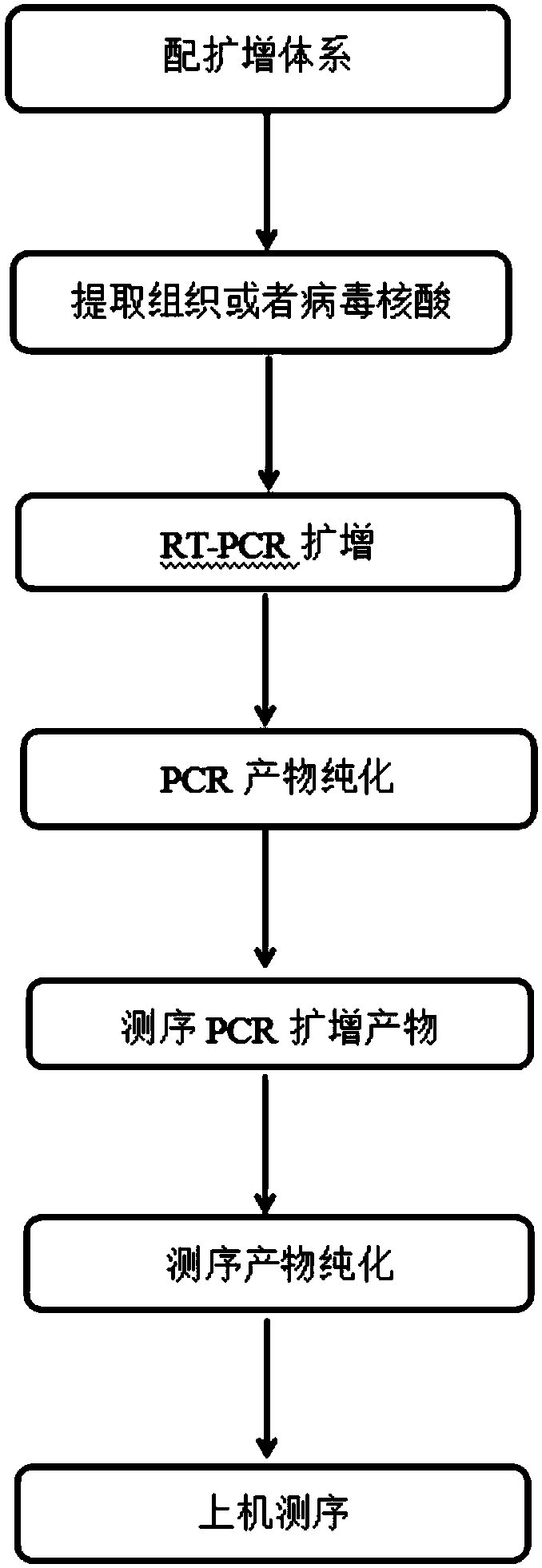
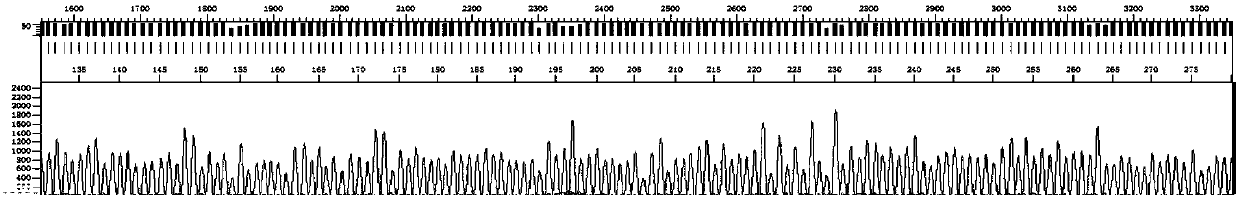
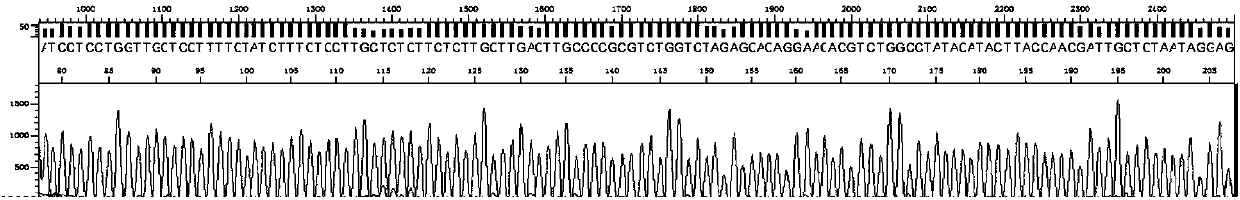
ActiveCN107653343AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesHepatitis C virus genotypeHuman hepatitis C virus
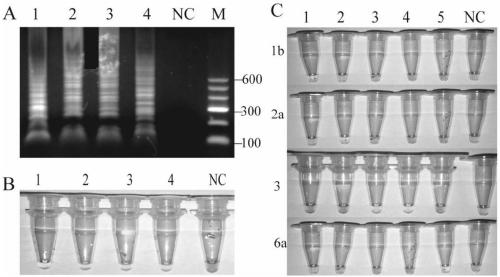
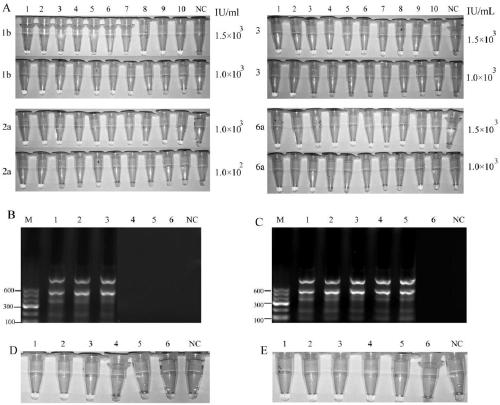
The invention discloses an amplification primer, a sequencing primer, a kit and a method for detection of hepatitis C virus genotyping. The amplification primer has the base sequences shown as SEQ IDNo:1 and SEQ ID No:2, and the sequencing primer has the base sequence shown as SEQ ID No:3. The method for detection of hepatitis C virus genotyping provided by the invention adopts the originally created primers for amplification and sequencing, and can be used for detection of common genotypes 1b, 2a, 3a, 3b and 6a of human hepatitis C virus.
Owner:英潍捷基(上海)贸易有限公司
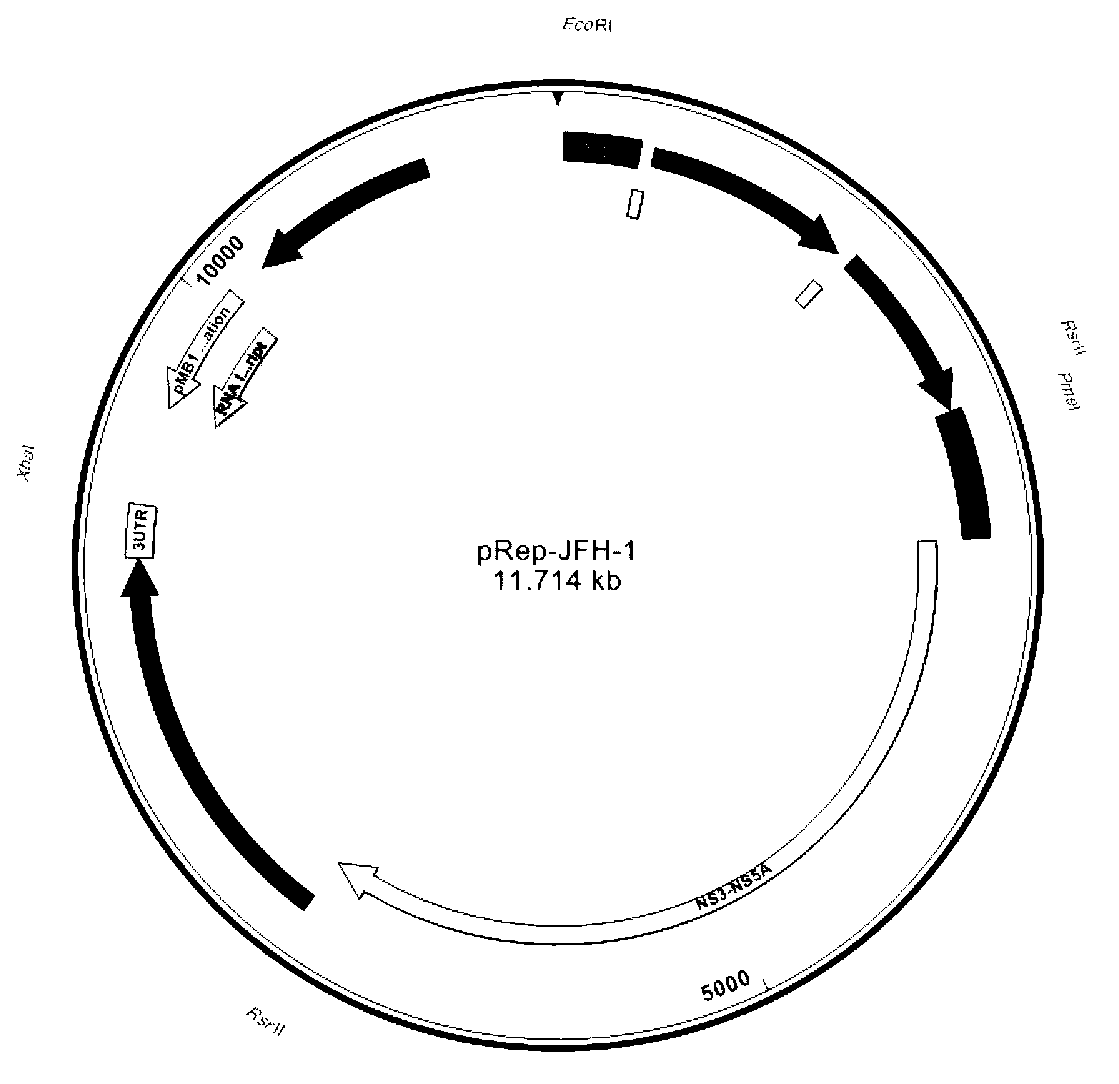
Hepatitis C virus chimeric replicon and its construction method
InactiveCN103215283AImprove replication efficiencyHigh efficiency of transient transfectionMicroorganism based processesFermentationHepatitis C virus genotypeStructural protein
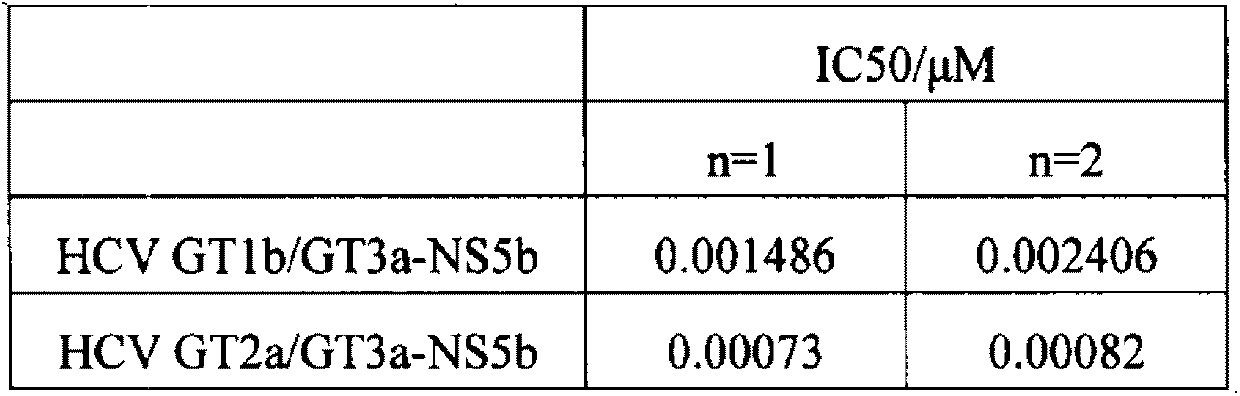
The invention provides a hepatitis C virus chimeric replicon and its construction method. The hepatitis C virus (HCV) chimeric replicon is a chimeric replicon of HCV genotype 2a and genotype 3a, and non-structural protein 5b, and is named as HCV GT2a / GT3aNS5b. The construction method includes: taking pRep-JFH-1 as the basis, and replacing its NS5B gene with GT3a NS5B gene, thus obtaining an NS5B chimeric replicon. Compared with the existing chimeric replicons taking GT1b as the skeleton, the hepatitis C virus chimeric replicon provided in the invention has high transient transfection efficiency, and the replication efficiency of the replicon does not need to be improved by constructing a stale cell line. Thus, the invention provides a rapid technical route for construction of more HCV clinical sample chimeric replicons in the future.
Owner:辉源生物科技(上海)有限公司
A kind of amplification primer, sequencing primer, kit and method for detecting hepatitis C virus genotyping
ActiveCN107653343BStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesMedicineHepatitis C virus genotype
The invention discloses an amplification primer, a sequencing primer, a kit and a method for detection of hepatitis C virus genotyping. The amplification primer has the base sequences shown as SEQ IDNo:1 and SEQ ID No:2, and the sequencing primer has the base sequence shown as SEQ ID No:3. The method for detection of hepatitis C virus genotyping provided by the invention adopts the originally created primers for amplification and sequencing, and can be used for detection of common genotypes 1b, 2a, 3a, 3b and 6a of human hepatitis C virus.
Owner:英潍捷基(上海)贸易有限公司
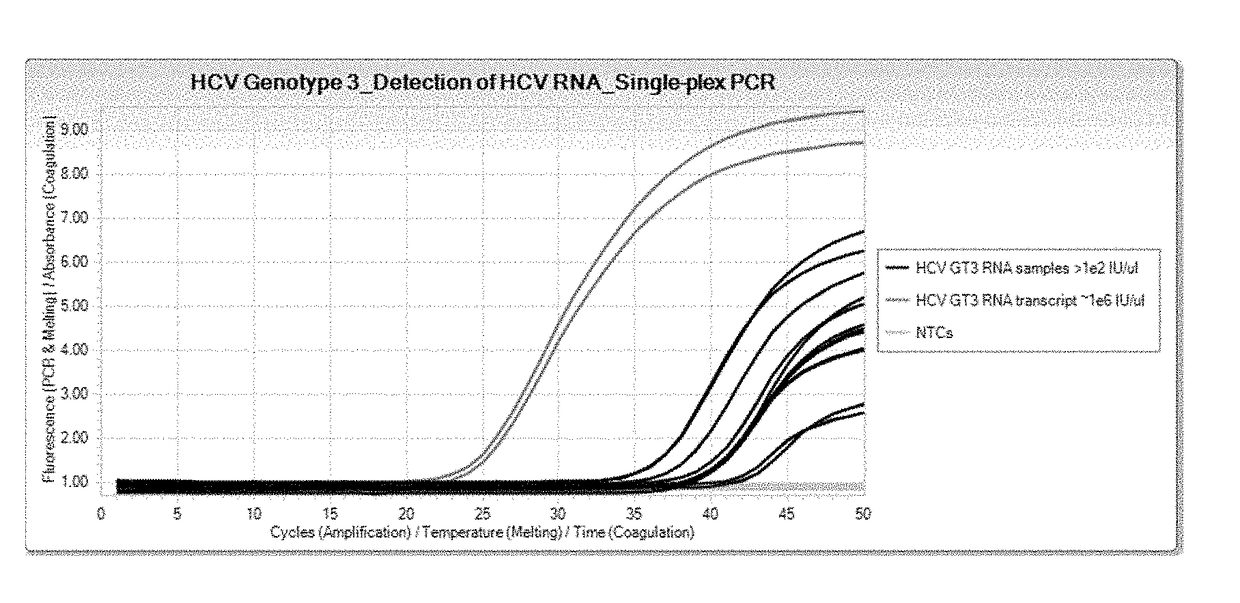
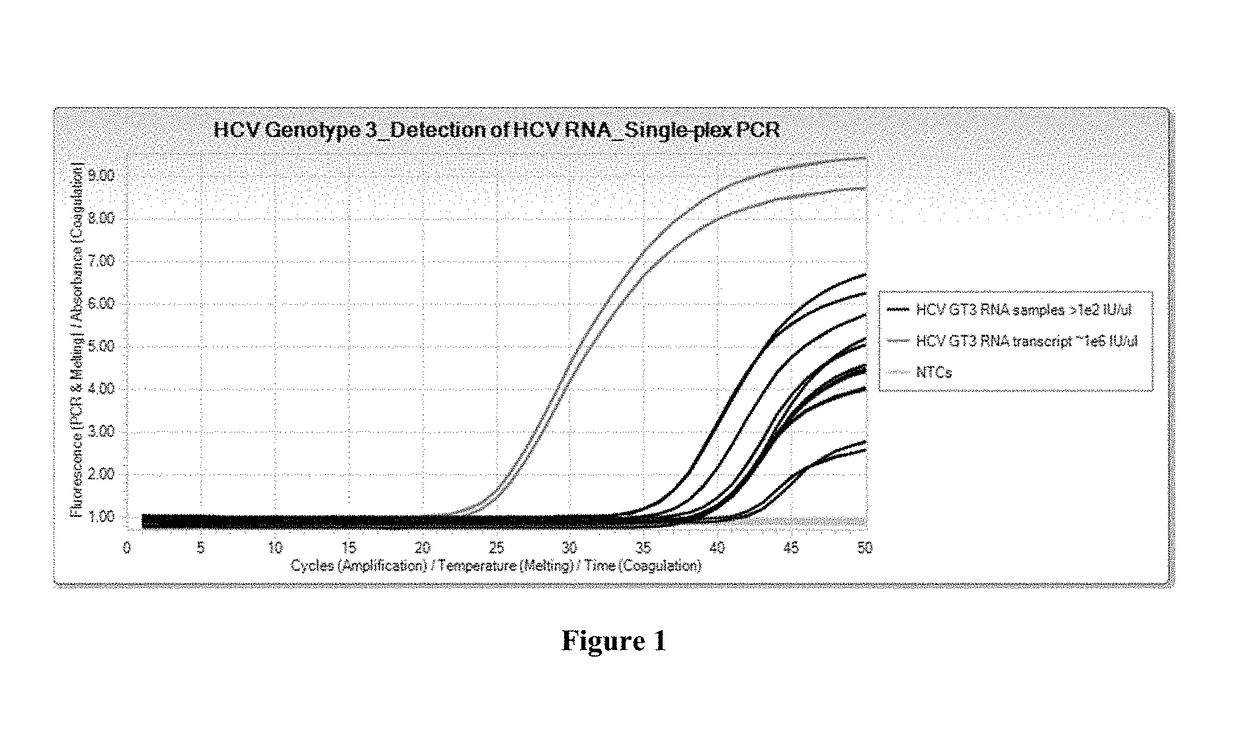
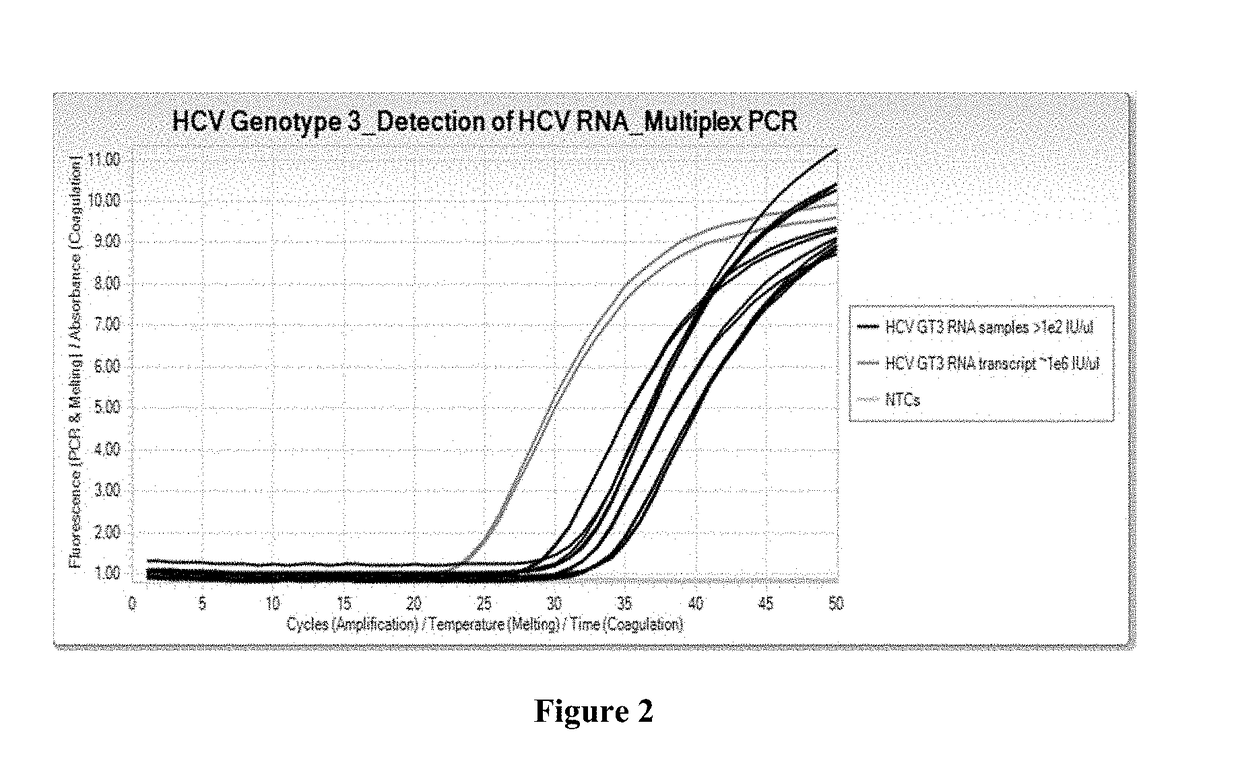
Compositions and methods for detection of hepatitis c virus genotype 3
ActiveUS20170356058A1Alter nucleic acid hybridization stabilitySsRNA viruses positive-sensePeptide/protein ingredientsHepatitis C virus genotypeHepacivirus
Methods for the rapid detection of the presence or absence of Hepatitis C Virus (HCV) in a biological or non-biological sample are described. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Furthermore, primers and probes targeting HCV and kits are provided that are designed for the detection of HCV.
Owner:ROCHE MOLECULAR SYST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Nucleic acid construct including nucleic acid derived from genotype 3[alpha] HCV genome Nucleic acid construct including nucleic acid derived from genotype 3[alpha] HCV genome](https://images-eureka.patsnap.com/patent_img/ed520ef3-17b0-4a37-b2b9-2b0f74946878/273136.PNG)
![Nucleic acid construct including nucleic acid derived from genotype 3[alpha] HCV genome Nucleic acid construct including nucleic acid derived from genotype 3[alpha] HCV genome](https://images-eureka.patsnap.com/patent_img/ed520ef3-17b0-4a37-b2b9-2b0f74946878/305414.PNG)
![Nucleic acid construct including nucleic acid derived from genotype 3[alpha] HCV genome Nucleic acid construct including nucleic acid derived from genotype 3[alpha] HCV genome](https://images-eureka.patsnap.com/patent_img/ed520ef3-17b0-4a37-b2b9-2b0f74946878/442181.PNG)