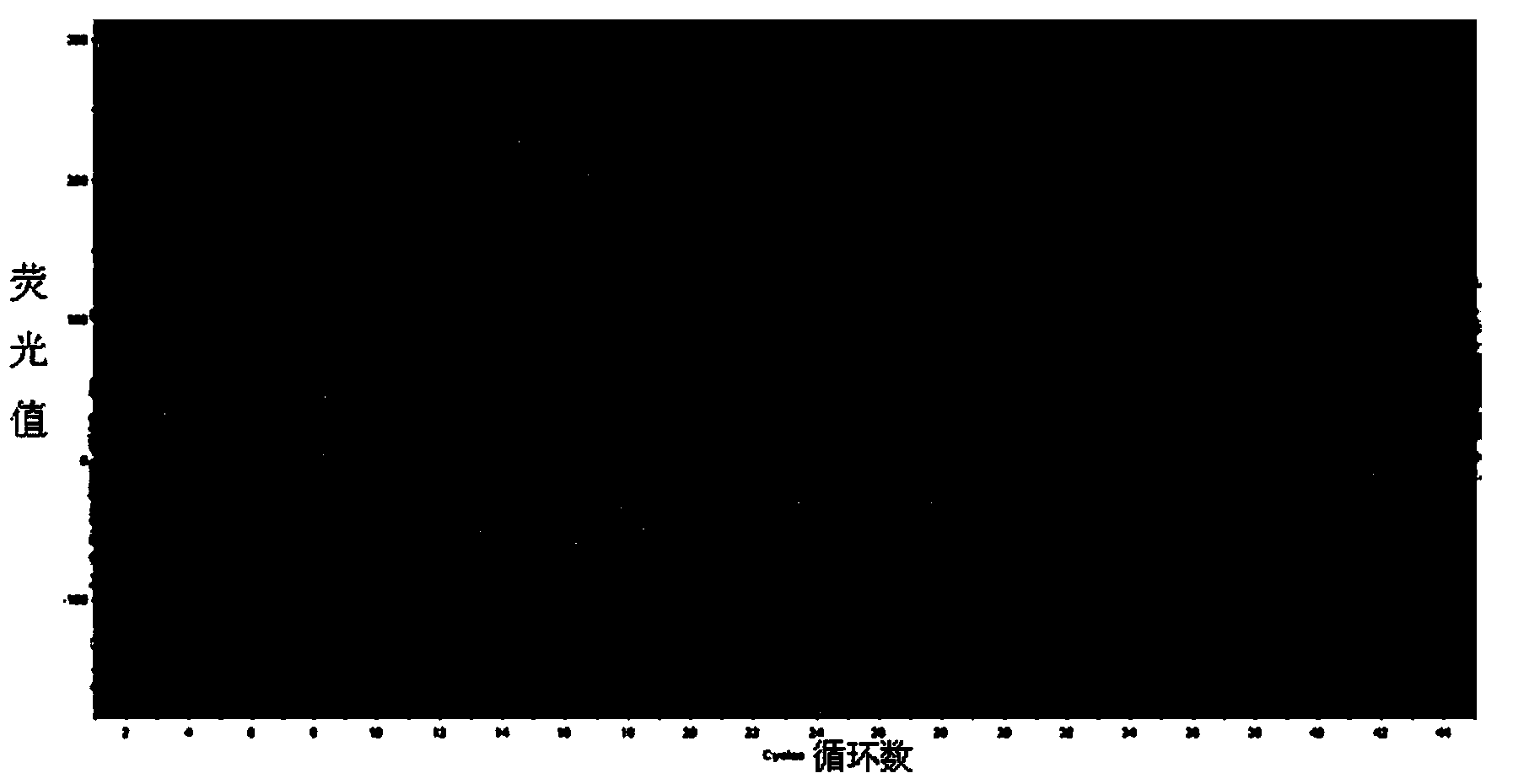
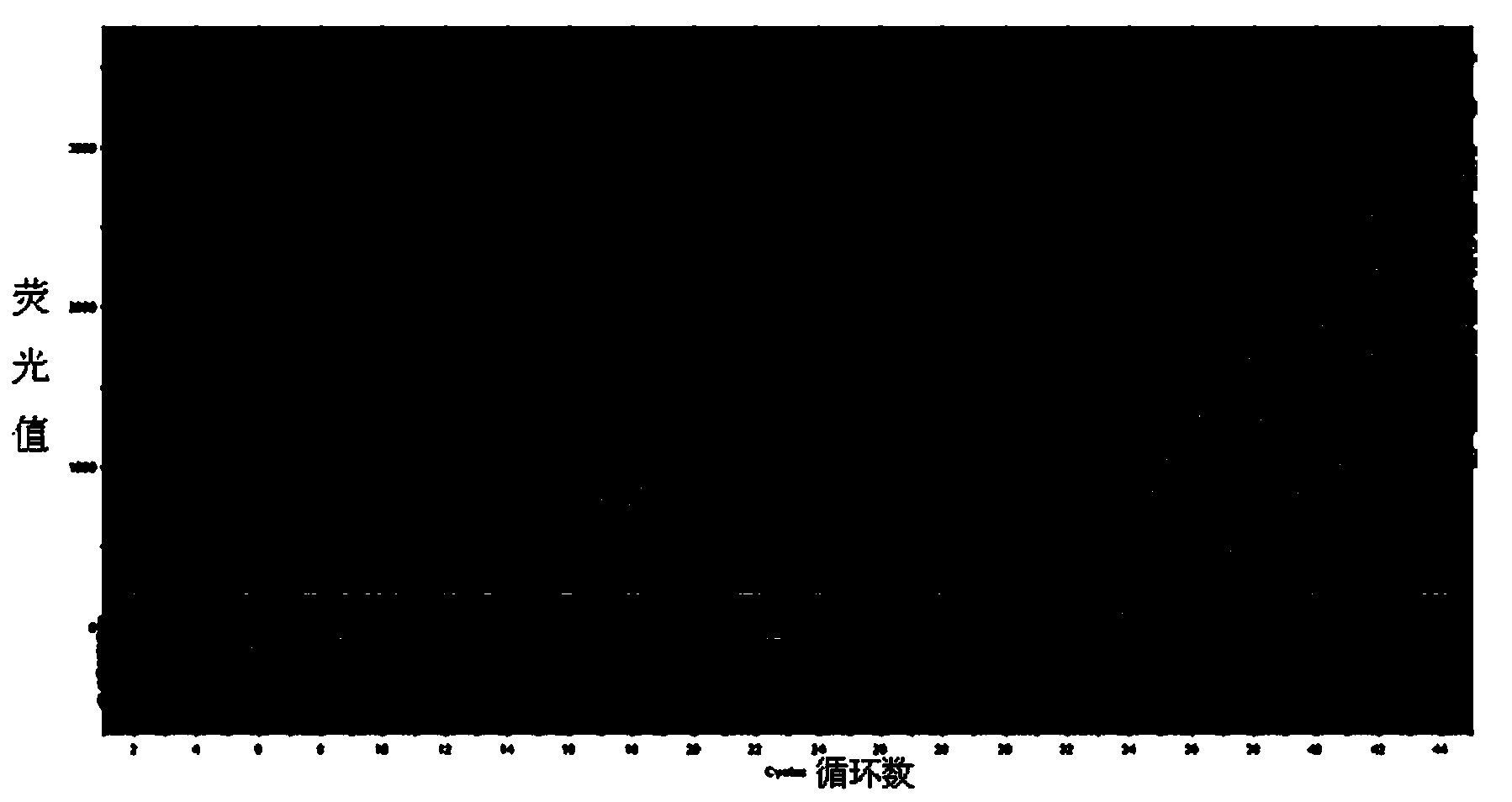
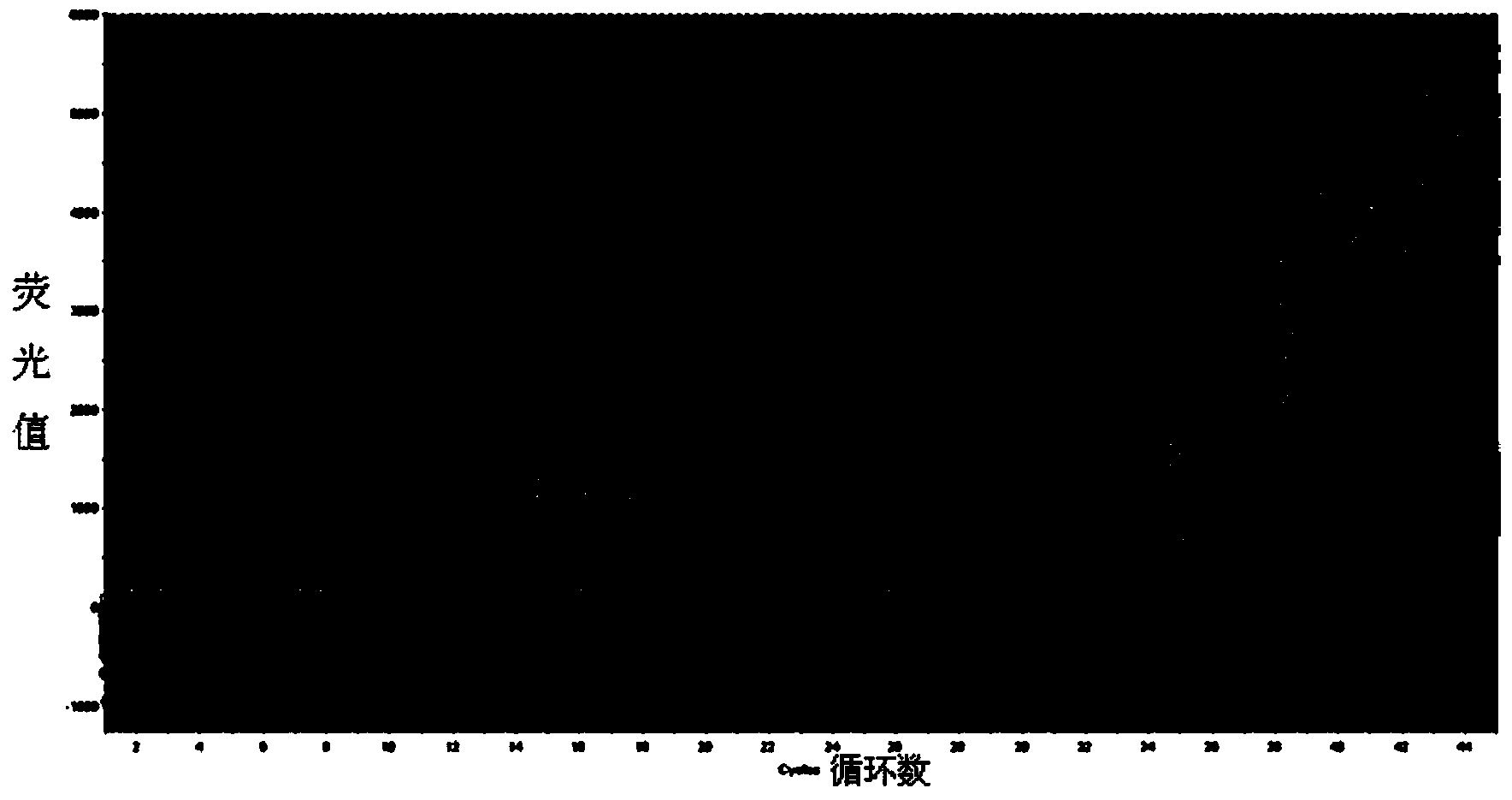
HCV (Hepatitis c virus) genotype detection kit
A kind of technology of hepatitis C virus, detection kit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1: provide a kind of HCV genotype detection kit
[0091] A HCV genotype detection kit, which consists of at least the following independent components:
[0092] HCV type 1 and type 3 PCR reaction solution: 10 μl of 5×PCR reaction buffer (included with Tth polymerase), 0.2 mmol / L deoxyribonucleoside triphosphate, 0.2 μmol / L~0.4 μmol / L for target polynucleoside Upstream and downstream primers HCV1-F, HCV1-R, HCV3-F, HCV3-R for acid amplification, 0.2μmol / L~0.4μmol / L probes HCV1-P, HCV3-P for target polynucleotide detection , the base pair sequences of the upstream and downstream primers used for target polynucleotide amplification and the probes used for target polynucleotide detection are respectively:
[0093] Upstream primer HCV1-F: 5'-AGGAAGACTTCCGAGCGGTC-3';
[0094] Downstream primer HCV1-R: 5'-TGCCATAGAGGGGCCAAGG-3';
[0095] Probe HCV1-P: 5'FAM-TACCCGGGCTGCGCCCAGG-BHQ13';
[0096] Upstream primer HCV3-F: 5'-GTCCTTTCTTGGAACAACCCGC-3';
[0097] Downs...
Embodiment 2
[0114] Embodiment 2: provide a kind of HCV genotype detection kit
[0115] A kind of HCV genotype detection kit, it also contains following several independently existing components except containing each component existing independently in embodiment 1:
[0116] Internal standard (positive internal control): a recombinant of a 100-base-pair artificially synthesized DNA sequence inserted into the pUC18T vector, that is, a plasmid, with a concentration of 1.00E+03IUs / ml~1.00E+06IUs / ml; 100 bases The sequence of base pairs is as follows:
[0117] 5'-CACCACTTAAATCCTAAGGTTCCAGCTCTGTCATCCAGTTTTGCTGACTCACGTATTCGTAGCAATCTTCTGGAGGTGCAATCTCAATTATGTCATCAG-3'
Embodiment 3
[0118] Embodiment 3: provide a kind of HCV genotype detection kit
[0119] A kind of HCV genotype detection kit, it also contains following several independently existing components except containing each component existing independently in embodiment 2:
[0120] HCV typing enzyme mixture: Tth enzyme 10U / μl~150U / μl, 1U / μl~10U / μl H-Taq DNA polymerase;
[0121] HCV typing positive control: calibrate the pseudovirus of known concentration, the concentration is 1.00~5.00E+05IU / ml.
[0122] HCV typing negative control: sterile saline.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com