Core antigen fragment for detecting hepatitis C virus and application of core antigen fragment
A technology for hepatitis C virus and core antigen is applied in the field of detecting the core antigen fragment of hepatitis C virus, which can solve the problems of reduced detection sensitivity of specimens, cross-contaminated aerosols, and reduced detection sensitivity, and achieves perfect detection sensitivity and accuracy. , the effect of making up for the lack of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
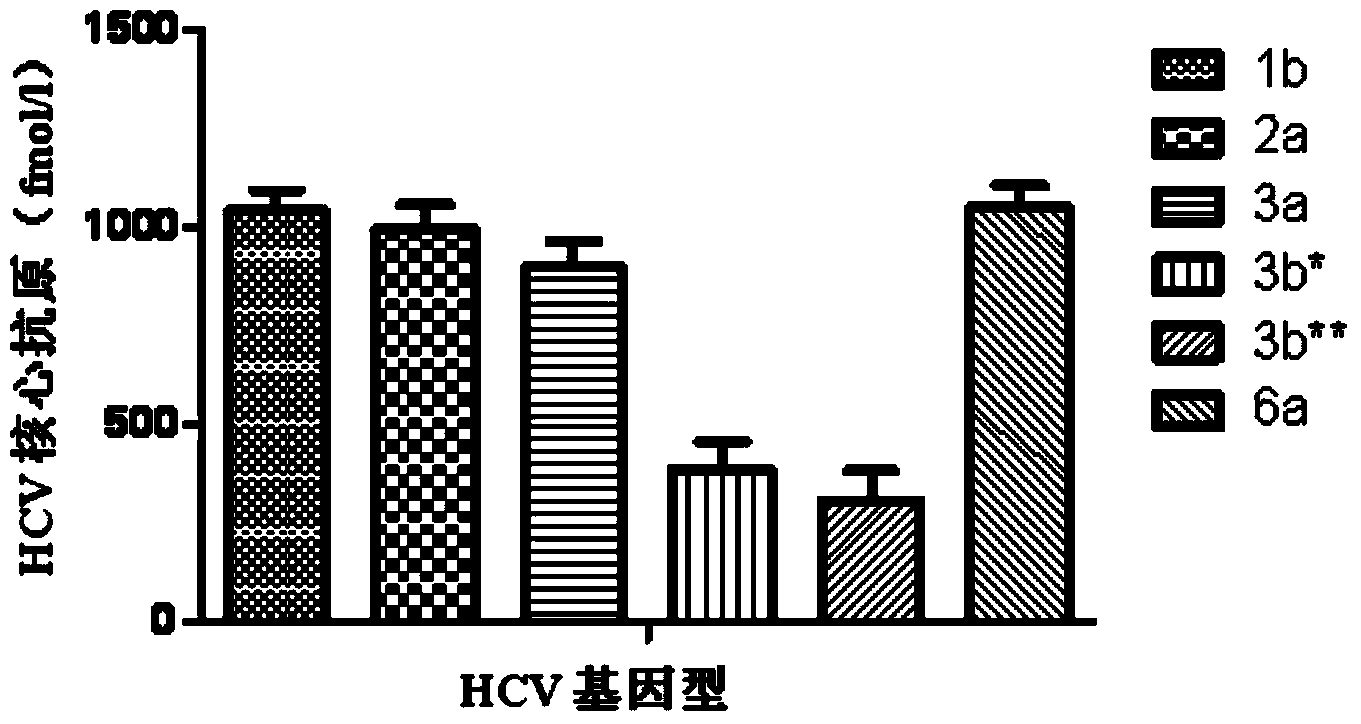
[0029] Example 1 Comparison of core antigen levels in samples of different HCV genotypes
[0030] 1. Case selection
[0031] From March to June 2011, sera from 203 CHC patients were collected, including 130 males and 73 females, aged 24-76 years, with an average of (37.3±8.6) years old. HCV genotypes were tested using Versant HCV Genotype 2.0 (LiPA, Siemens, Marburg, Germany), among which genotype 1b was 41 cases, 2a was 43 cases, 3a was 31 cases, 3b was 43 cases, and 6a was 45 cases. Covers common genotypes in China. HCV RNA was quantified using Roche Taqman HCV RNA kit, with a minimum detection limit of 15IU / mL. Patients with different HCV genotypes are comparable in age, gender and HCV viral load.
[0032] 2. Method
[0033] Use Abbott Architect HCV Ag Core Antigen Quantitative Detection Kit to perform HCV antigen quantitative detection on Abbott Architect i2000 automatic chemiluminescence analyzer. The linear range of quantitative detection is 3.00fmol / L-20000fmol / L, and the l...
Embodiment 2
[0038] Example 2 Comparison of the sensitivity of Abbott Architect HCV Ag Quantitative Kit for detecting HCV samples of different genotypes
[0039] 1. Sample selection Select 1 high viral load serum of 1b, 2a, 3a, 3a and 6a genotypes.
[0040] 2. Experimental method Use Abbott Realtime HCV RNA quantification kit to detect the viral load of each genotype sample, and then dilute each genotype serum so that the final concentration of HCV RNA is 10000, 7500, 5000, 1000, 750, 500, 250, respectively And 100IU / mL. Dilute samples of different concentration levels were tested 20 times with Abbott Architect HCV Ag quantitative reagent. Using the probability analysis method (Probit method), calculate the lowest HCV RNA concentration when 95% of the samples are “positive” by the Abbott Architect antigen quantitative detection. This concentration is regarded as the sensitivity of the Architect HCV Ag quantitative kit, which is the “lowest” Detection limit".
[0041] 3. Experimental results
[...
Embodiment 3
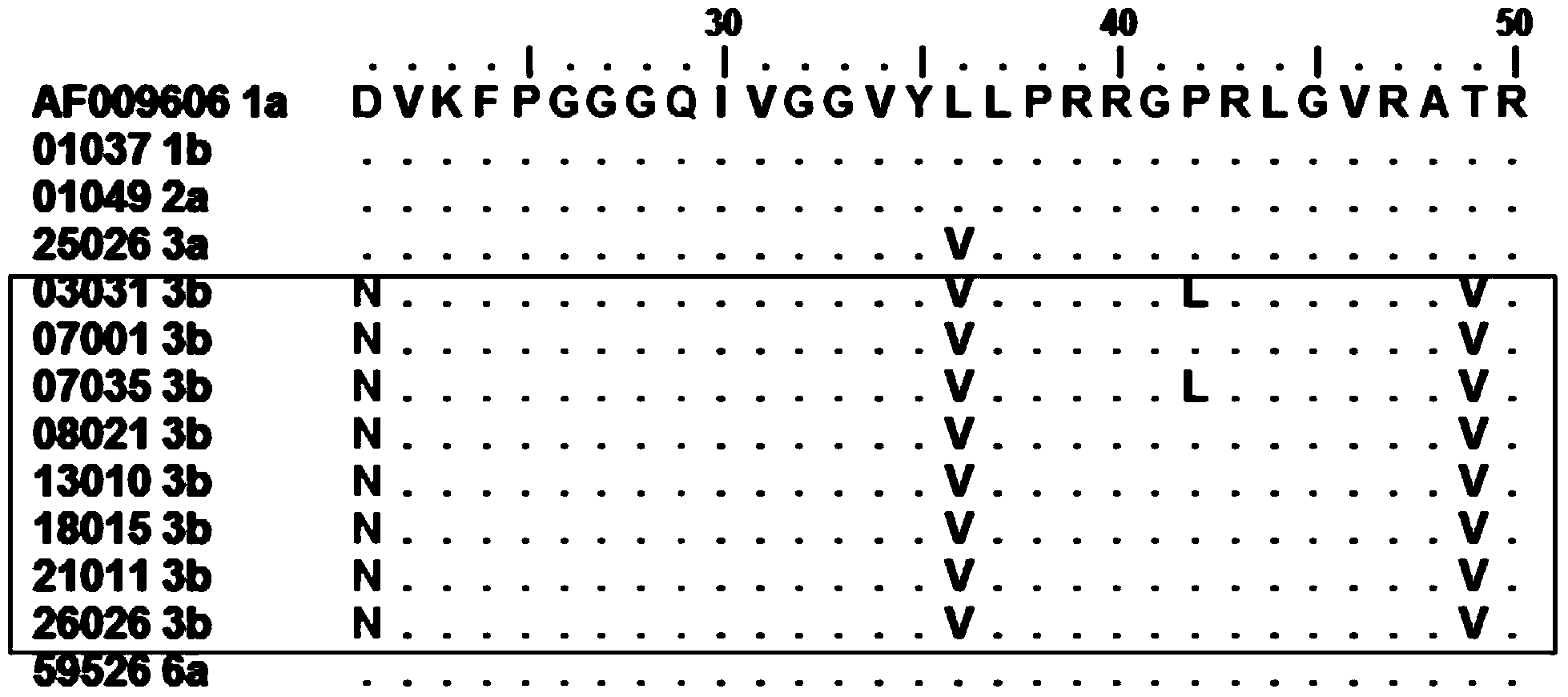
[0045] Example 3 Alignment of Amino Acid Sequences in the Core Region of HCV Strains of Different Gene Subtypes
[0046] 1. Materials and methods
[0047] (1) Serum HCV RNA extraction
[0048] Use the QIAamp virus RNA extraction kit from QIAGEN, Germany to extract HCV RNA from 140 μl serum.
[0049] (2) Reverse transcription and PCR amplification (RT-PCR) of HCV core region
[0050] Reverse transcription of HCV RNA was performed using AMV reverse transcriptase from Promega, USA. The total volume of one RT reaction system is 10μl, including 6μl HCV RNA, 1×AMV reaction buffer, 1mM each dNTP, 3μM RT primer Core410R and 6U AMV reverse transcriptase. The RT reaction conditions are: 42°C, 60 min. Add 40μl PCR reaction solution to 10μl 5’-UTR reverse transcription system to make the total volume of PCR reaction system 50μl, containing 2.5U Platinum Taq DNA polymerase from Invitrogen, 1×PCR buffer, 1.5mMMg 2+ , 200μM each dNTP, 0.6μM primer Core406F. PCR conditions are: 95°C, 5min for DNA d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com