New treatments of hepatitis c virus infection
A hepatitis C virus, standard therapy technology, used in the treatment of patients with hepatitis C virus genotype 2 and 3 infection, pharmaceutical use of cyclophilin inhibitors in the treatment of hepatitis C virus infection, Alisporivir field, Able to address severe depression, significant, leukopenia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0106] 1. Compound
[0107] Peg-IFNα2a is a pegylated form of interferon alpha-2a and utilizes a 40 kDa branched PEG (polyethylene glycol) to provide sustained serum concentrations throughout the week (168 hours). Commercially available from Roche.
[0108] Ribavirin is a synthetic nucleoside analogue and can also be purchased, for example, from Roche
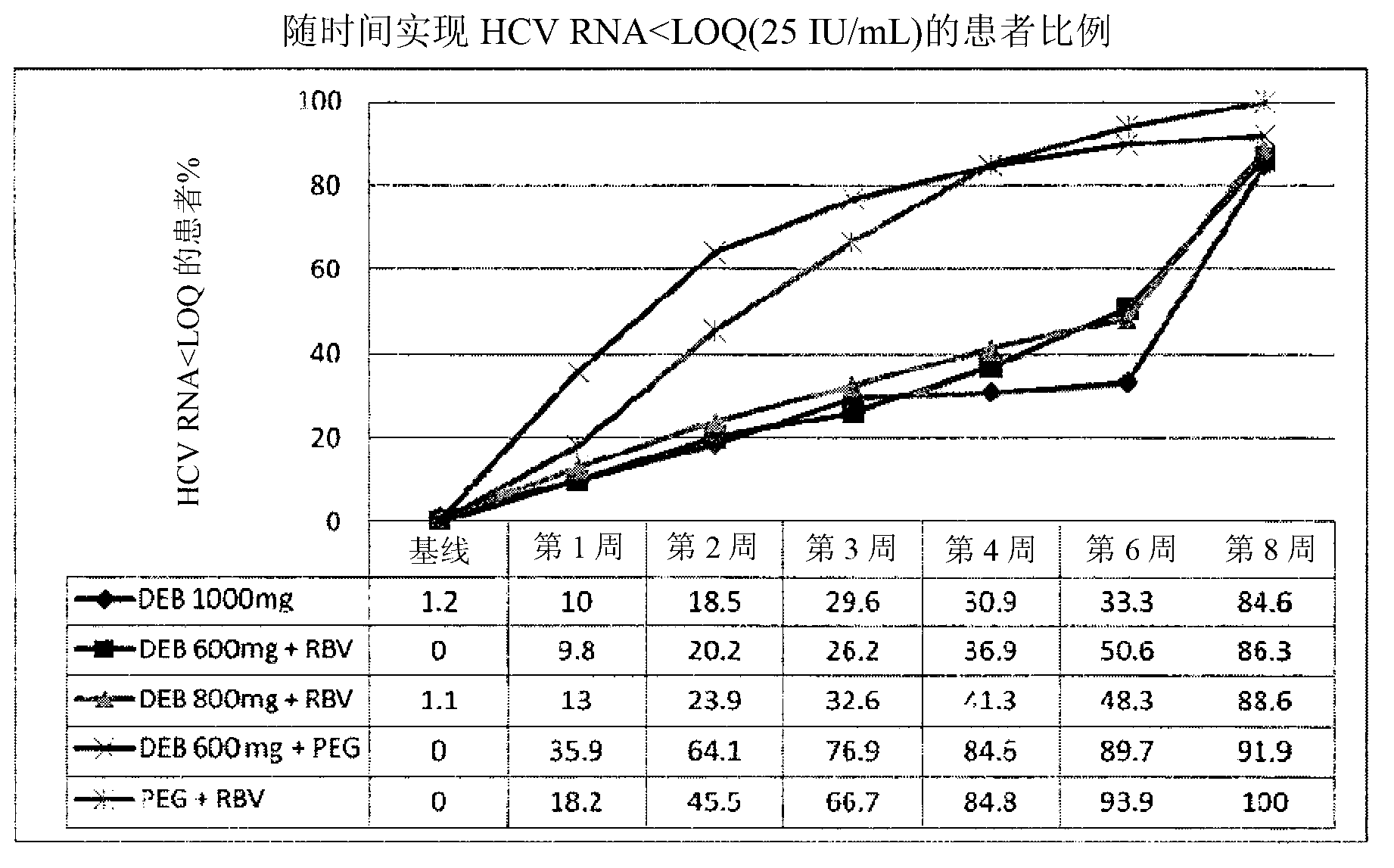
[0109] 2. Clinical trials and results
[0110] An international, multicentre, randomized, parallel-group, open-label, multiple-dose phase II study.
[0111] Patients were randomly assigned in a 2:2:2:1:1 ratio to one of the 5 treatment groups (A, B, C, D and E) as described below.
[0112] Therapy A
[0113]Alisporivir: Oral 3 alisporivir capsules 200 mg (600 mg) 2× / day (twice daily or BID) for one week, followed by 5 200 mg alisporivir capsules (1000 mg) 1× / day (once a day or QD ) for 23 weeks.
[0114] therapy B
[0115] Alisporivir: Oral 3 alisporivir capsules 200mg (600mg) 2x / day for one week, followed by 3 alispo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com