Expression vector PVX-6His-CTBt-Bt for producing multi-epitope vaccine of hepatitis c virus
A technology of pvx-6his-ctbt-bt and 6his-ctbt-bt, which is applied in the field of expression vectors for the production of hepatitis C (hepatitis C) virus multi-epitope vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
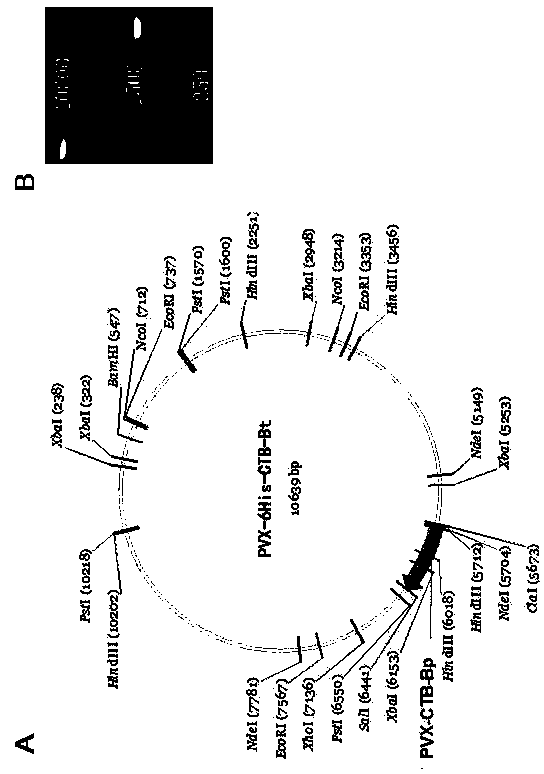
[0033] The construction and identification of PVX-6His-CTBt-Bt carrier, its method is as follows:
[0034] (1) Vaccine amino acid composition design:
[0035] Amino acid composition design of hepatitis C hepatitis virus multi-epitope vaccine: 6 histidines from N-terminus to C-terminus (to facilitate vaccine purification), oral immune adjuvant cholera toxin B subunit CTB (enhance the oral immune effect of the vaccine), Six B-cell neutralizing epitopes of Bt (E1 313-327aa -E2 396-424aa -E2 438-447aa -E2 523-540aa -E2 610-627aa -E2 631-648aa ). Each epitope is separated by a glycine and a serine residue).
[0036] The amino acid sequence of the hepatitis C virus vaccine 6His-CTBt-Bt designed according to the above requirements is:
[0037] His His His His His His His Met Ile Lys Leu Lys Phe Gly Val Phe Phe
[0038] 1 5 10 15
[0039]Thr Val Leu Leu Ser Ser Ala Tyr Ala His Gly Thr Pro Gln Asn Ile
[0040] 20 25 30
[0041] Thr Asp Leu Cys Ala Glu Tyr His Asn Thr Gln ...
Embodiment 2
[0150] Vaccine expression and Western blot identification in tobacco, its method is as follows:
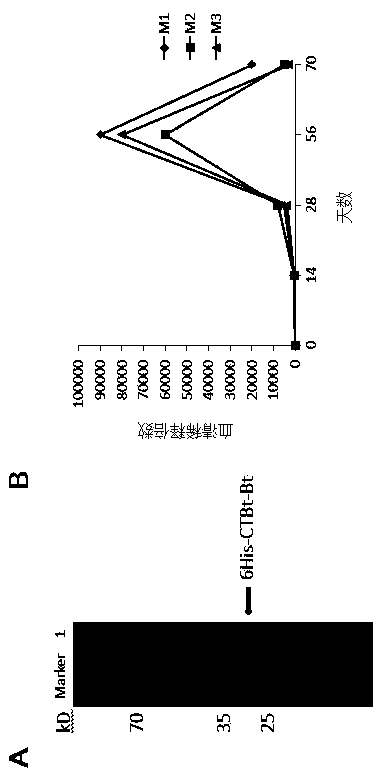
[0151]Corundum worn tobacco leaves were inoculated with the vaccine expression vector PVX-6His-CTBt-Bt. At the same time, potato X virus PVX201 (negative control) and PVX204 (carrying GFP gene) were inoculated with tobacco leaves as controls, and the amount of virus vector inoculated on each leaf was 2 -4μg. To verify the expression of GFP protein, to optimize the effect of virus inoculation and replication, to prepare the protein extract of tobacco, and to use mouse-derived 6×His Tag antibody (LanPower?) and CTB antibody (antibodies-online Inc), respectively, for Western blot Detection of vaccine expression. Ni column purification of expressed vaccine protein 6His-CTBt-Bt.
[0152] According to the experimental method described above, the recombinant expression vector PVX-6His-CTBt-Bt was inoculated into tobacco. At the same time, the positive control PVX204 (expressing GFP gene...
Embodiment 3
[0154] Animal experiments are used to determine the immune characteristics and safety of vaccines, and the methods are as follows:
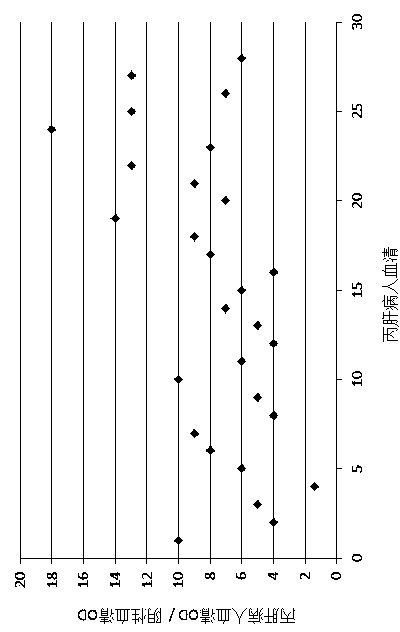
[0155] Four-week-old BALB / c mice were orally immunized with the vaccine protein purified by Ni column three times (immunization time was 1, 14 and 28 days), and each time each mouse was immunized with 10 μg of vaccine protein. Rats immunized with tobacco leaf protein extract (10 μg) without vaccine served as controls. Three mice were immunized in the experimental group and the control group respectively. Blood was collected at different times, serially diluted 2 times, and antibody titers were determined at different periods (1, 14, 28, 56 and 70 days) by ELISA. Under the condition that the OD value (490nm) of the positive serum is at least 2 times greater than the OD value of the negative control (rat serum before vaccine immunization and mouse serum not immunized with vaccine), the maximum dilution of positive serum is the titer of this antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com