Hepatitis C virus genotyping method based on loop-mediated isothermal amplification
A technology of hepatitis C virus and genotyping method, which is applied in the direction of biochemical equipment and methods, microorganism measurement/inspection, etc. It can solve the problems of high laboratory pollution risk, easy amplification failure, high equipment requirements, etc., and achieve low Cost, High Sensitivity, Effect of High Sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A hepatitis C virus genotyping method, based on the LAMP method, downloads the sequences of HCV subtypes from GeneBank (CHINA HCV 1b:L02836.1, HQ912959.1, HQ912956.1, GU451224.1, HCV 2a:HQ639945. 1, KC844043.1, HQ639944.1, HQ639938.1, KF676352.1, HCV 3: AP008209.2, HQ912953.1, KC844041.1, KC844044.1, HCV 6a: DQ480524.1, HQ912955.195, HQ9 1, KC844037.1, DQ480516.1), using Geneious10.2.3 software to compare the sequences of each subtype, select the specific and conserved sequence segment of each subtype in the 5'UTR-Core region, and use primer explorer V.5 (http: / / primerexplorer.jp / e / ) software to design various types of LAMP reaction primers, including two outer primers (F3, B3), two inner primers (FIP, BIP) and loop primers (LF or LB); Primers were synthesized by Sangon Bioengineering Co., Ltd. (Shanghai, China).
[0052] The reaction system includes: 12.5μL 2×MasterMix (6mM MgSO4, 1.4mM dNTP Mix, 320U Bst3.0DNA Polymerase), 2.5μL primer mixture (40pmol each for FIP an...
Embodiment 2
[0054] A method for genotyping hepatitis C virus 1b, 2a, 3 and 6a comprising:
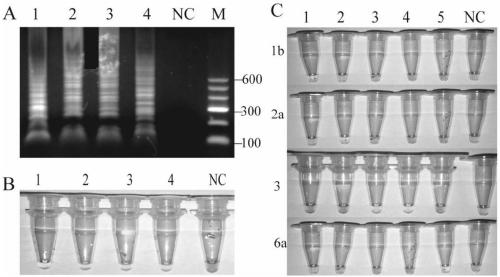
[0055] Serum samples: A total of 108 serum samples were collected from the First Affiliated Hospital of Kunming Medical University (Yunnan, China) and Kunming Infectious Disease Hospital (Yunnan, China) from August to December 2017 after clinical testing. Among them, 80 specimens were identified by gene sequencing method to determine the subtype of HCV, and the markers of hepatitis A, hepatitis B, HIV and cytomegalovirus were negative, which were used to evaluate the specificity of the method; 10 cases of hepatitis B virus serum, 10 cases of HIV virus serum, 8 cases of hepatitis A virus, and negative hepatitis C markers, used to verify the anti-interference of RT-LAMP method.
[0056] Nucleic acid extraction: Serum samples were extracted using a DNA / RNA magnetic bead method nucleic acid extraction kit (Tianlong Technology Co., Ltd., Xi'an, China), and the nucleic acid extraction method was operated...
Embodiment 3
[0068] Hepatitis C virus genotyping method based on embodiment 2
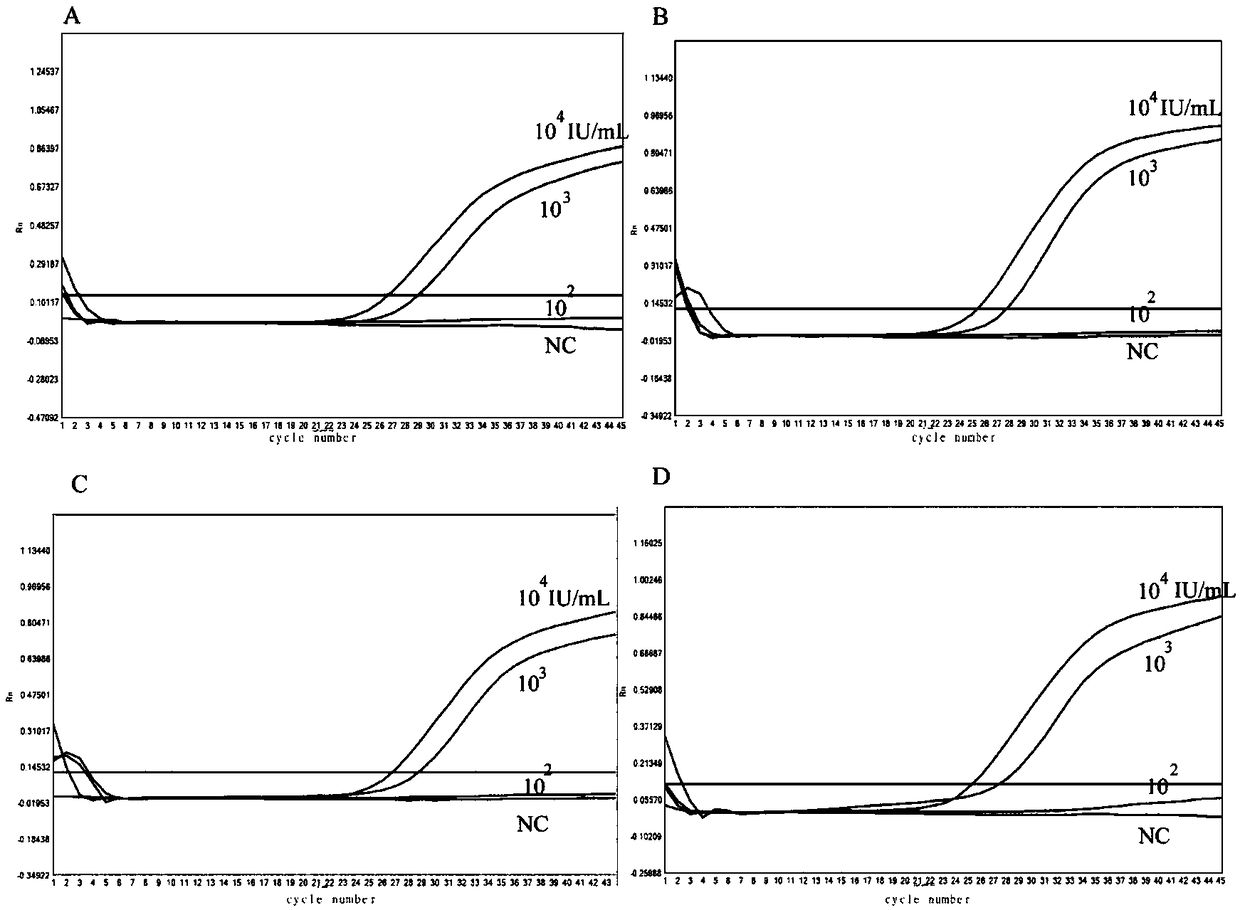
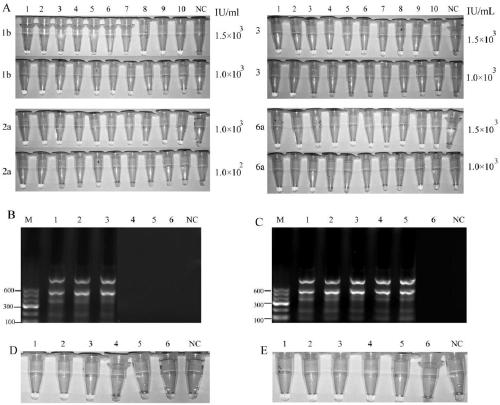
[0069] Evaluation of the lowest detection limit: HCV1b, 2a, 3, 6a type RNA (concentration is about 1.0×10 6 IU / mL) specimens were diluted with 10-fold gradient with HCV-negative serum to obtain a series of samples (1.0 × 10 6 ,1.0×10 5 ,1.0×10 4 ,1.5×10 3 ,1.0×10 3 ,1.0×10 2 IU / mL), and then divide the samples of each concentration into 10 parts to extract nucleic acid respectively. The above samples were tested by both LAMP and Real-time PCR methods at the same time, and the lowest concentration of all 10 samples positive was regarded as the lowest detection limit.
[0070]The lowest detection limit of HCV-1b, 2a 3 and 6a by LAMP method and Real-time PCR.
[0071] Such as figure 2 as shown ( figure 2 : Evaluation of the lowest detection limit of HCV-1b, 2a, 3 and 6a by LAMP method. A: LAMP method for detection of HCV-1b, 2a, 3 and 6a reaction chart, 1-10 represents 10 repeated reaction wells of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com