Octreotide composition based on subcutaneous gel slow release, preparation method and application
A technology of octreotide and composition, applied in the field of biopharmaceuticals, can solve the problems of inconvenient administration and inability to meet the needs of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
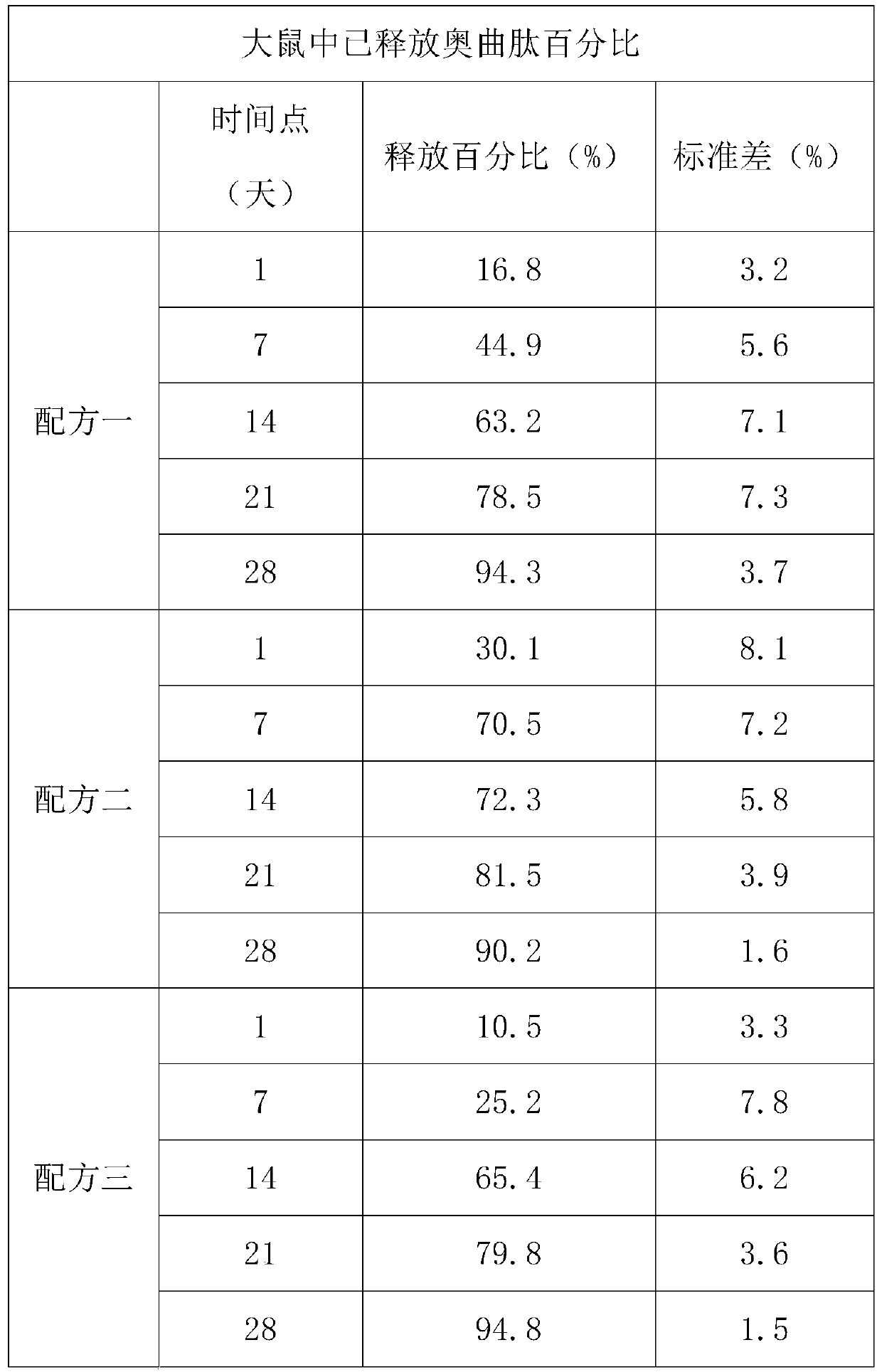
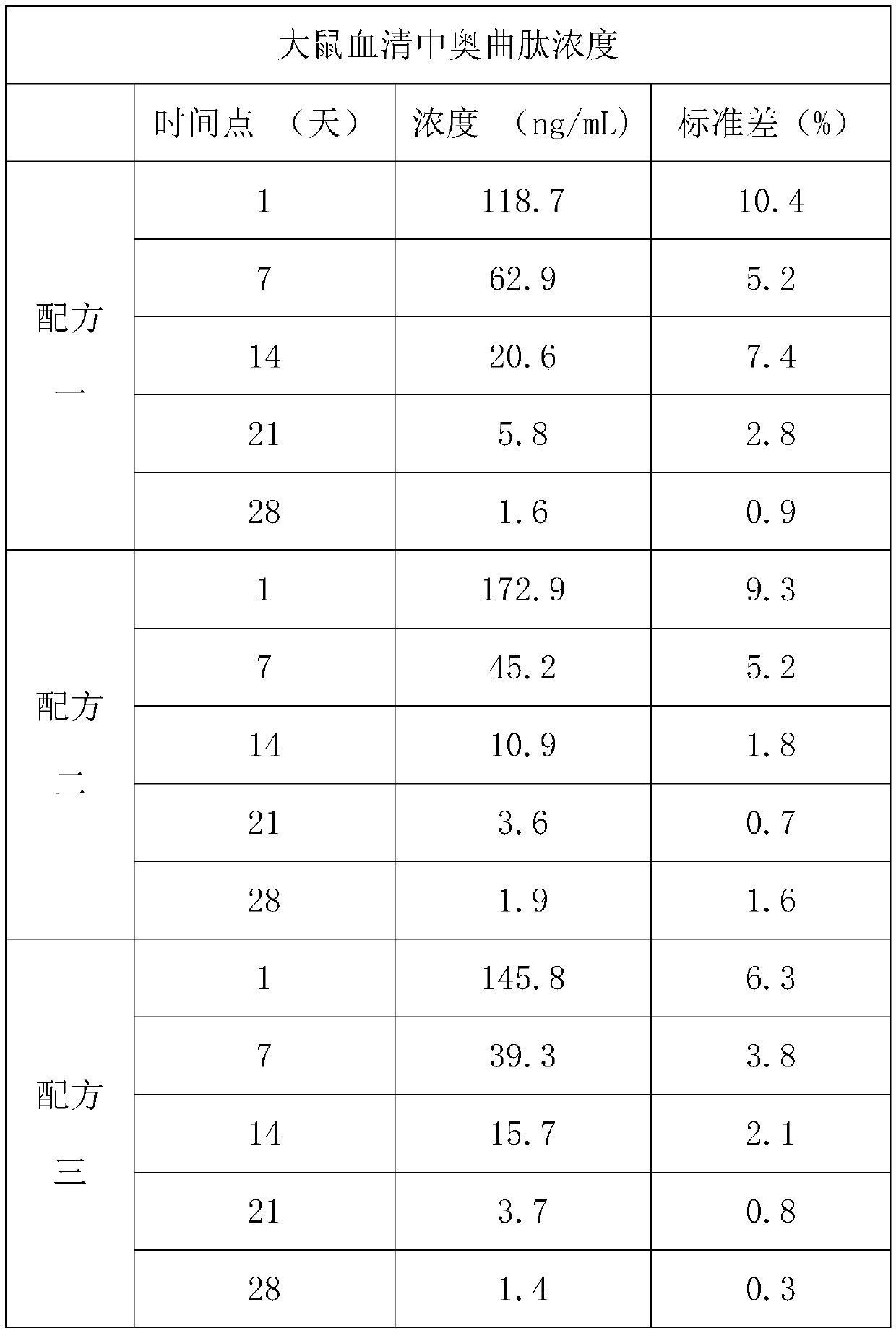
[0019] Example: Study on release kinetics of octreotide subcutaneous gel formulation in rats
[0020] The main objective of this study was to evaluate the efficacy of three different gel formulations in large 28-day release kinetics in rats. Another goal was to collect blood for plasma concentration analysis of octreotide. The ultimate goal is to evaluate the tissue response of the test site and the tissue compatibility of the test object from a macro perspective.
[0021] In the 28-day study, three subcutaneous gel formulations were tested in 120 male rats. On day 0, four gel formulations (approximately 200 microliters) containing 10 mg of octreotide citrate were injected subcutaneously in the abdomen per rat. On days 1, 7, 14, 21, and 28, rats were anesthetized and bled by cardiac puncture. The plasma concentration of octreotide was analyzed by liquid chromatography / mass spectrometry / mass spectrometry (LC / MS / MS). The subcutaneous residue was removed and subsequently ana...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap