A carbonylated polycaprolactone-based photodynamic therapy prodrug and its preparation method and application
A technology of polycaprolactone and carbonylation, which is applied in the field of carbonylated polycaprolactone-based photodynamic therapy prodrugs and preparations, can solve problems such as instability and poor fat solubility of ALA, and achieve high conversion rate and good photodynamic therapy Curative effect, good targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of CABALA:
[0042]
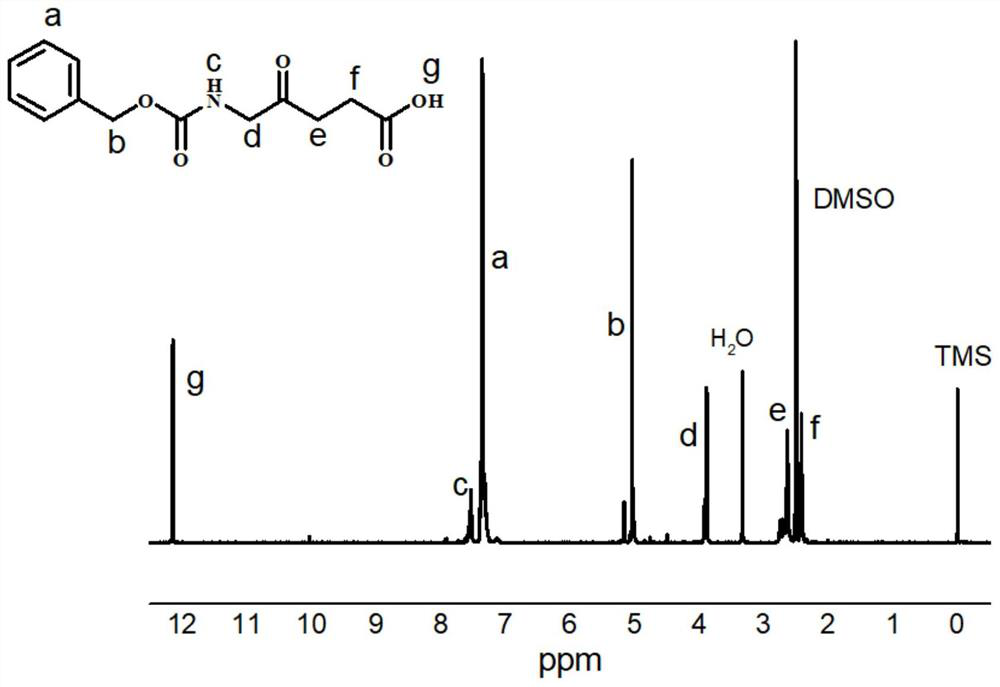
[0043]Add 1.68g of 5-aminolevulinic acid hydrochloride, 0.84g of sodium bicarbonate and 20ml of deionized water in sequence in a 50mL two-necked flask. After the above raw materials are completely dissolved, add 1.7g of benzyl chloroformate dropwise in an ice-water bath , reacted at room temperature overnight, concentrated and filtered to remove unreacted benzyl chloroformate and solvent, the resulting white solid was washed with methanol and dried to constant weight to obtain the compound CABALA. figure 1 is the compound CABALA 1 H NMR spectrum.
Embodiment 2
[0045] Synthesis of Polycaprolactone-Based Amphiphilic Copolymer mPEG-b-P(CL-co-OPD)
[0046]
[0047] x=20, y=30.
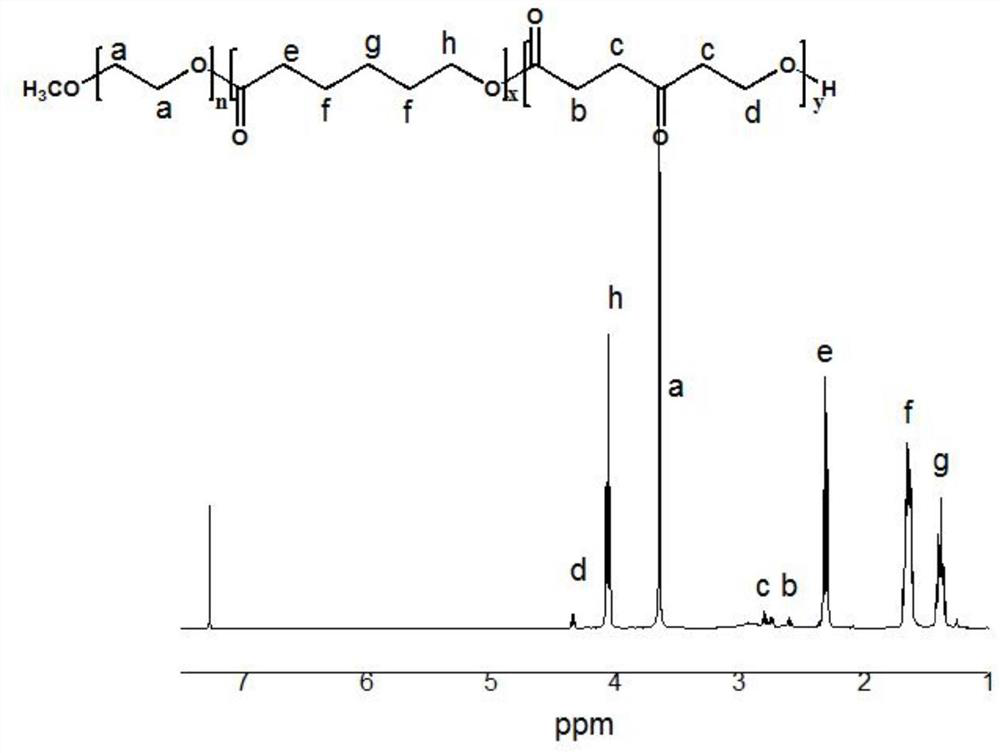
[0048] The 25mL reaction eggplant bottle was evacuated three times, and under the protection of argon, 1g of polyethylene glycol monomethyl ether 2000 (mPEG 2000), 1.28g of 4-carbonyl-ε-caprolactone (OPD) and 1.71g of ε- Caprolactone (ε-CL), vacuumize for 2h, add 10ml of dry toluene and catalyst Sn(Oct) 2 0.101g, reacted at 90°C for 24h. The reaction product was settled in glacial ether, and the sediment was suction-filtered with a sand core funnel, and vacuum-dried to constant weight to obtain polycaprolactone-based amphiphilic copolymer mPEG-b-P (CL-co-OPD). figure 2 It is a polycaprolactone-based amphiphilic copolymer mPEG-b-P (CL-co-OPD) 1 H NMR spectrum.
[0049] The preparation method of carbonylated polycaprolactone-based photodynamic therapy prodrug mPEG-b-P (CL-co-ALAOPD) comprises the following steps:
[0050]
[0051] x=20, y=30.
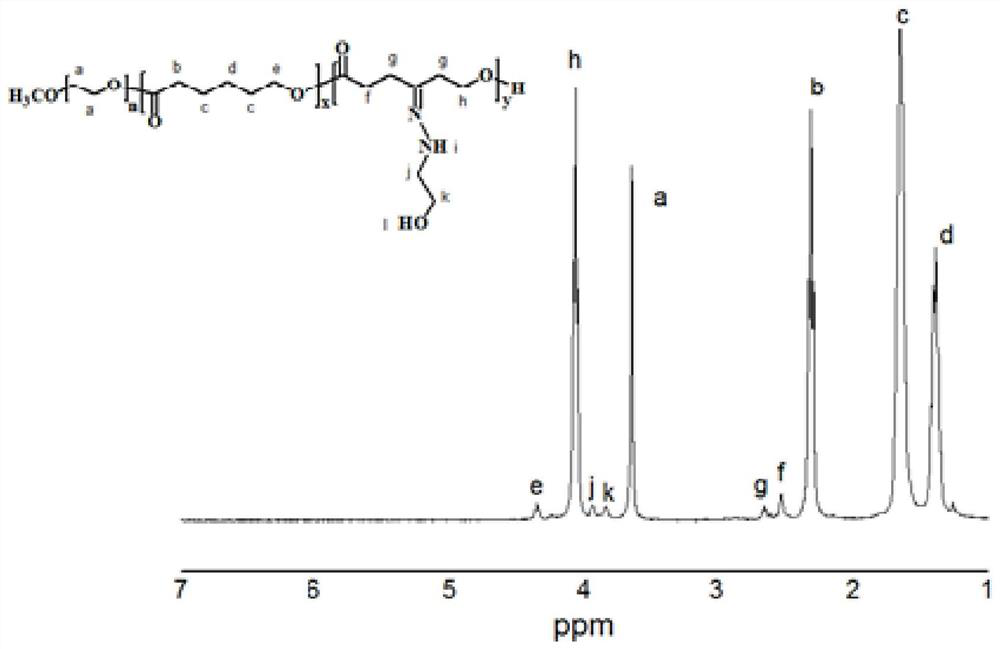
[0052...
Embodiment 3
[0057] Synthesis of Polycaprolactone-Based Amphiphilic Copolymer mPEG-b-P(CL-co-OPD)
[0058]
[0059] x=30, y=20.
[0060] The 25mL reaction eggplant bottle was evacuated three times, and under the protection of argon, 1g of polyethylene glycol monomethyl ether 2000 (mPEG 2000), 1.92g of 4-carbonyl-ε-caprolactone (OPD) and 1.14g of ε- Caprolactone (ε-CL), vacuumize for 2h, add 10ml of dry toluene and catalyst Sn(Oct) 2 0.101g, reacted at 90°C for 24h. The reaction product was settled in glacial ether, and the sediment was suction-filtered with a sand core funnel, and vacuum-dried to constant weight to obtain polycaprolactone-based amphiphilic copolymer mPEG-b-P (CL-co-OPD).
[0061] The preparation method of carbonylated polycaprolactone-based photodynamic therapy prodrug mPEG-b-P (CL-co-ALAOPD) comprises the following steps:
[0062]
[0063] x=30, y=20.
[0064] Access to hydrazine: Weigh 1g of polycaprolactone-based amphiphilic copolymer mPEG-b-P (CL-co-OPD) and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com