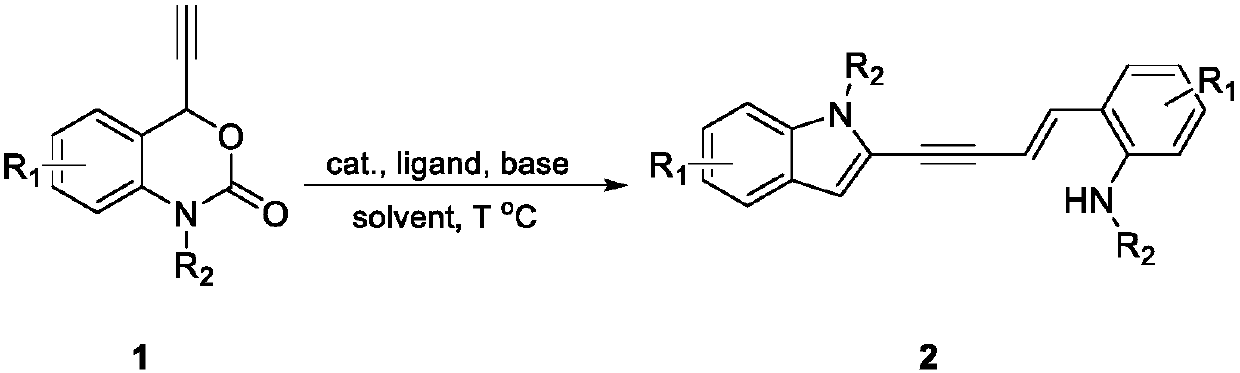
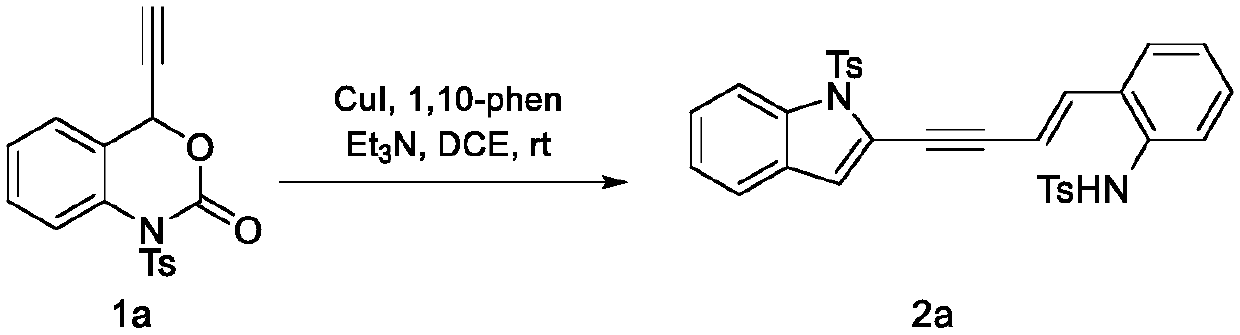
Method for preparing 4- (2 '-indolyl)-1, 3-eneyne derivative
A technology for indolyl and derivatives, which is applied in the field of preparing 4--1,3-enyne derivatives, can solve problems such as difficult to control regioselectivity, and achieves wide application range of substrates, cheap and easily available reagents, Simple to use effects
Active Publication Date: 2020-06-05
DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the substrate has many reaction sites, and it is easy to undergo intramolecular or intermolecular cycl
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a method for preparing a 4- (2 '-indolyl)-1, 3-enyne derivative. Specifically, 4-ethynyl-1, 4-dihydro-2H-benzo [d] [1, 3]-oxahexane-2-one is used as a raw material under the action of an alkali, and the 4- (2 '-indolyl)-1, 3-enyne derivative is constructed in one step through a two-molecule reaction catalyzed by a transition metal. The method has the advantages of cheap and easily available reagent, simple operation, wide substrate application range and the like.
Description
technical field [0001] The present invention relates to a method for preparing 4-(2'-indolyl)-1,3-enyne derivatives. Background technique [0002] In recent years, 4-ethynyl-1,4-dihydro-2H-benzo[d][1,3]-nitroxane-2-one compounds are often used in organic synthesis in copper-catalyzed systems , is an efficient synthon. However, the substrate has many reaction sites, and it is easy to undergo intramolecular cyclization or intermolecular cyclization under acid-base or metal catalysis, so it is difficult to control the regioselectivity of the reaction. This reaction provides a method for the selective synthesis of unsymmetrical enynes. Contents of the invention [0003] The object of the present invention is to provide a method for synthesizing 4-(2'-indolyl)-1,3-enynes. [0004] [0005] The specific operation steps are as follows: [0006] React in the reactor, add 4-ethynyl-1,4-dihydro-2H-benzo[d][1,3]-nitroxane-2-one (1), then add catalyst, compound Body, base and ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D209/14
CPCC07D209/14
Inventor 万伯顺杨萨娜钱磊磊易如霞吴凡
Owner DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com