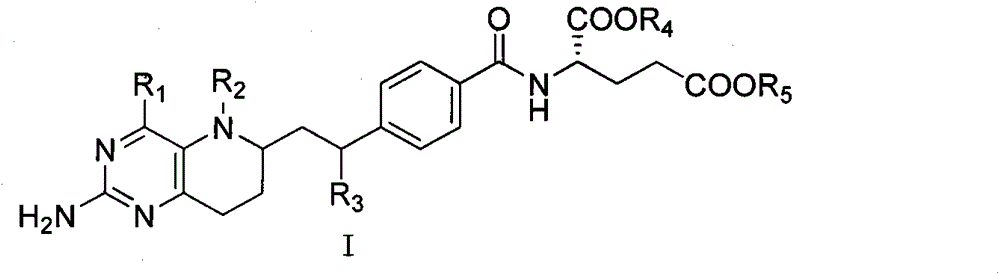
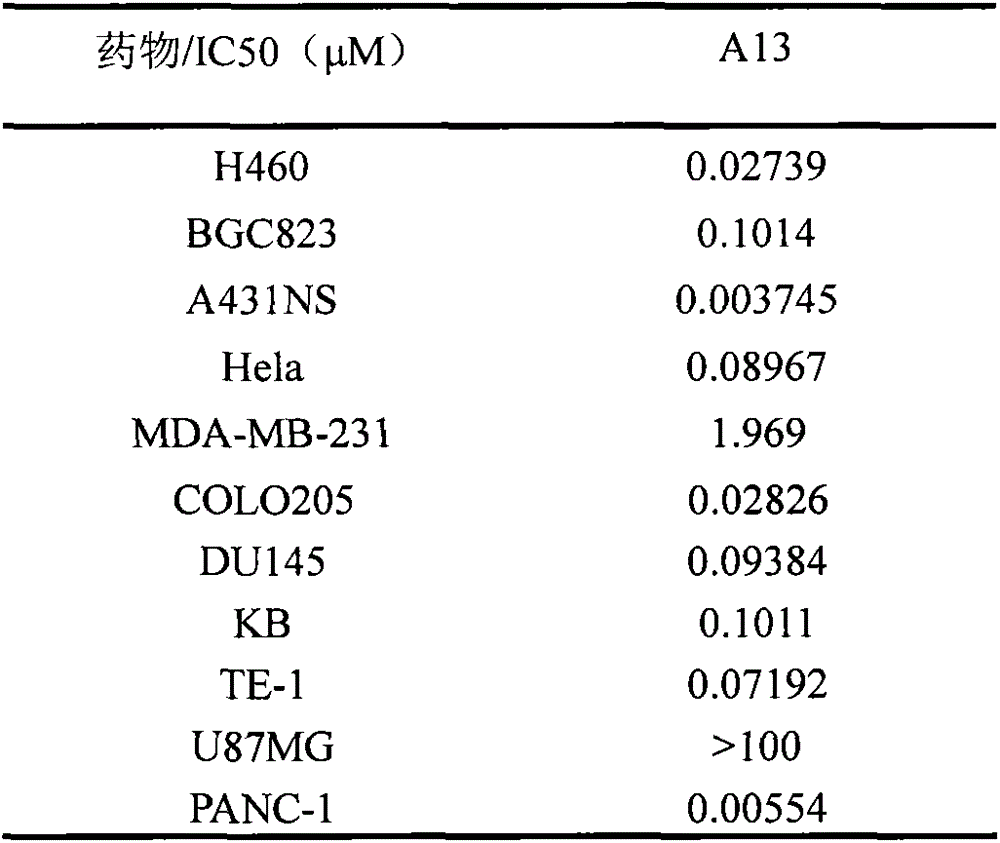
Application of novel 8,10-dediaza-N5-acyl tetrahydrofolic acid compound to antitumor drug
The technology of a compound and a drug is applied in the field of preparing a folic acid metabolism methionine synthase inhibitory effect, and achieves the effects of environmental friendliness, cheap and easily available reagents, and few synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
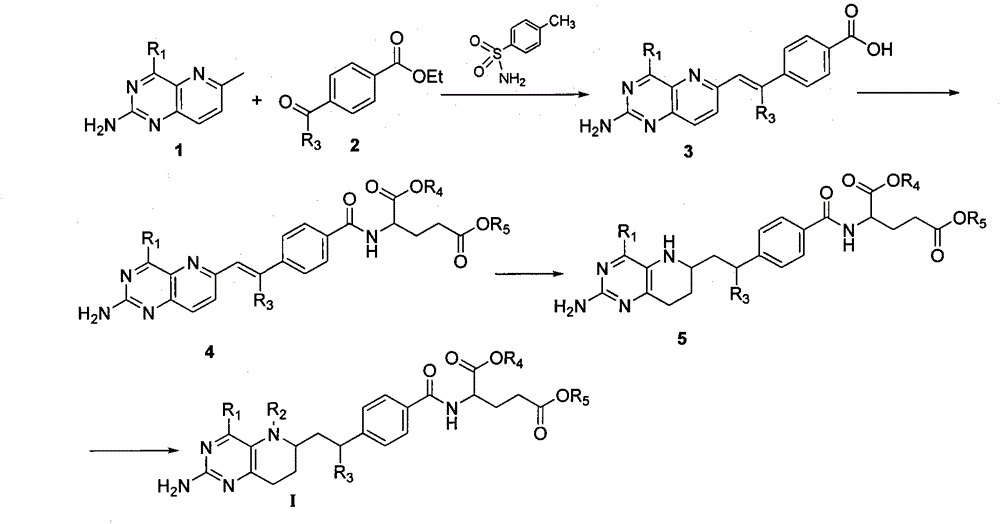
[0033] Example 1: Synthesis of (E)-4-[2-(2,4-diaminopyrido[3,2-d]pyrimidin-6-yl)vinyl]benzoic acid
[0034] Dissolve 1mmol 2,4-diamino-6-methylpyrido[3,2-d]pyrimidine (1), 1mmol ethyl p-formylbenzoate (2) and 1mmol p-toluenesulfonamide in 5mL N , in N-dimethylacetamide, heated to reflux for 12 h under nitrogen protection, TLC monitored the disappearance of the reaction raw materials, added an appropriate amount of 1N NaOH to the reaction solution to pH = 12, extracted and washed the solution with dichloromethane three times, collected the aqueous layer, 1N HCl was added to adjust the pH to 5, and a white solid was precipitated, which was filtered by suction and dried to obtain compound 3 with a yield of 90.5%. m.p.>250℃; 1 H NMR (DMSO-d 6 , 400MHz) δ: 6.37 (s, 2H, 2-NH2), 6.45 (d, J=16.0Hz, 1H, CH=CHPh), 7.57 (d, J=8.4Hz, 1H, CH-7, py), 7.74~7.78 (m, 2H, C 6 h 4 and 1H, CH-8, Py), 7.89 (d, J=16.0Hz, 1H, CH=CHPh), 7.94 (d, J=8.0Hz, 2H, C6H4), 11.0 (d, J=7.3Hz, 1H , COOH); ...
Embodiment 2
[0035] Example 2: (E)-4-[2-(2,4-diaminopyrido[3,2-d]pyrimidin-6-yl)vinyl]benzoyl-L-glutamic acid diethyl ester Synthesis
[0036] With 1mmol compound (E)-4-[2-(2,4-diaminopyrido[3,2-d]pyrimidin-6-yl)vinyl]benzoic acid, 1mmol diethyl glutamate and 1mmol 1 , 3-dicyclohexylcarbodiimide (DCC) was dissolved in dichloromethane, stirred at room temperature for 24h, TLC monitored the disappearance of the reaction raw materials, and the mother liquor was washed with CH 2 Cl 2 and saturated brine, and the organic layer was washed with anhydrous Na 2 SO 4 Distilled under reduced pressure after drying to a small amount, separated by column chromatography, and the eluent was CH 2 Cl 2 :CH 3 OH=15:1, 5 g of compound 1.9 was obtained, yellow-green fluorescent solid, yield 31.5%, m.p.133-134°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 1.160~1.204(m, J=7.2Hz, 6H, 2×CH 3 ), 2.019~2.058 (m, 1H, C H a h b CH 2 CO), 2.111~2.184 (m, 1H, CH a H b CH 2 CO), 2.448~2.485(t, 2H, CH2CO), 4.036~4.08...
Embodiment 3
[0037] Example 3: 4-[2-(2,4-diamino-5,6,7,8-tetrahydropyrido[3,2-d]pyrimidin-6-yl)ethyl]benzoyl-L - Synthesis of diethyl glutamate
[0038] 100mg (0.201mmol) (E)-4-[2-(2,4-diaminopyrido[3,2-d]pyrimidin-6-yl)vinyl]benzoyl-L-glutamic acid diethyl Dissolve the ester in 10mL absolute ethanol, add 2mg PtO 2 and 1mL HOAc, catalytic hydrogenation under the condition of 0.4MPa hydrogen, TLC monitoring, stop the reaction after about 120h, filter off PtO 2 , with 5% NaHCO 3 The aqueous solution was neutralized, and the ethanol was distilled off under reduced pressure, and CH 2 Cl 2 and saturated brine, and the organic layer was washed with anhydrous Na 2 SO 4 After drying, it was distilled under reduced pressure to a small amount, separated by column chromatography, and the eluent was V(CH2Cl2):V(CH3OH)=12:1 to obtain 80 mg of compound 4-[2-(2,4-diamino-5,6 , 7,8-tetrahydropyrido[3,2-d]pyrimidin-6-yl)ethyl]benzoyl-L-glutamic acid diethyl ester, light yellow solid, yield 79.0%.m.p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com