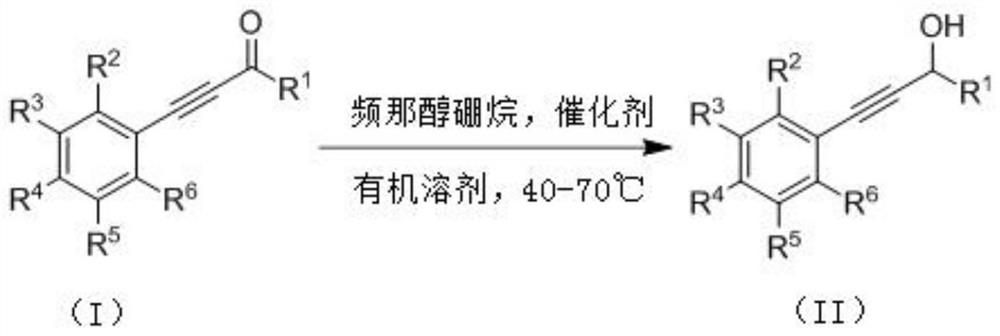
Method for preparing propargyl alcohol compound by taking pinacol borane as hydrogen source
A technology of pinacol borane and propargyl alcohol, applied in the field of chemistry, can solve the problems of unsatisfactory substrate applicability and poor functional group tolerance, and achieve simple method, mild reaction conditions and strong atom economy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
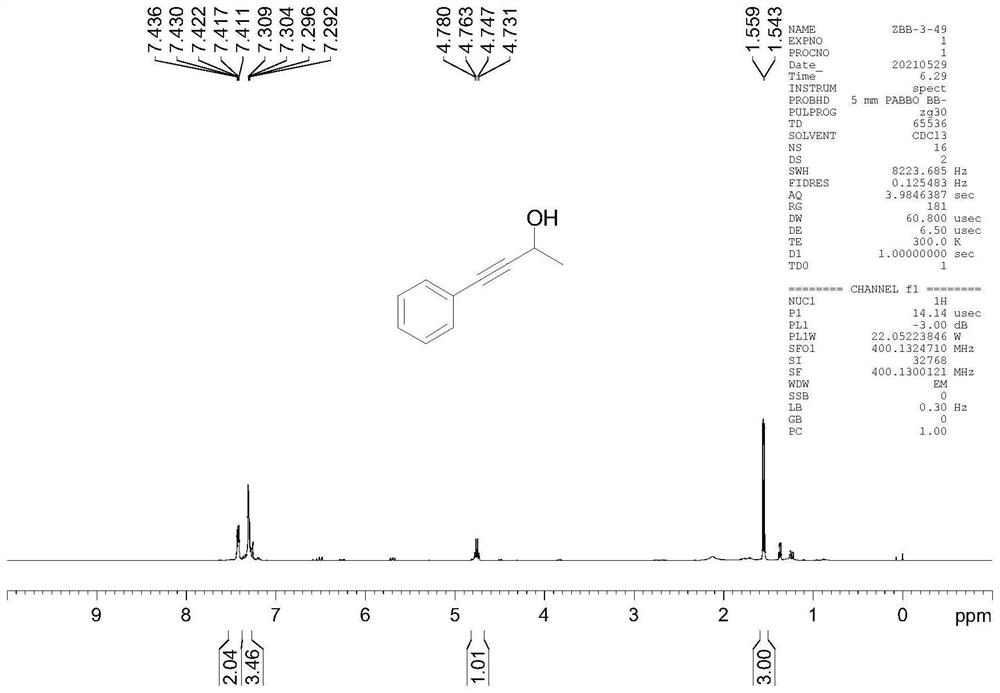
[0041] Example 1 Preparation of 4-phenyl-3-butyn-2-ol
[0042]
[0043] In a water-free and oxygen-free glove box under argon atmosphere 4 NBr (0.02 mmol) was added to a 10 mL reaction tube, then 2 ml THF (tetrahydrofuran), then pinacol borane (1.0 mmol) was sent out of the glove box, and then 4-phenyl-3-butyne- 2-Keto (0.2 mmol). Reaction in 60°C oil bath, TLC monitoring and I 2 The reaction was detected by color development. After the reaction was completed, it was quenched by adding sodium hydroxide solution (15mol / L), extracted and concentrated, and carried out silica gel column chromatography with petroleum ether / ethyl acetate as eluent to obtain 4-phenyl-3 -butyn-2-ol. Yellow oily liquid, yield 94%. 1 HNMR (400MHz, CDCI 3 ) 7.44-7.41, (m, 2H), 7.31-7.29 (m, 2H), 4.78-4.73 (m, 1H), 1.56-1.54 (m, 1H).
Embodiment 2
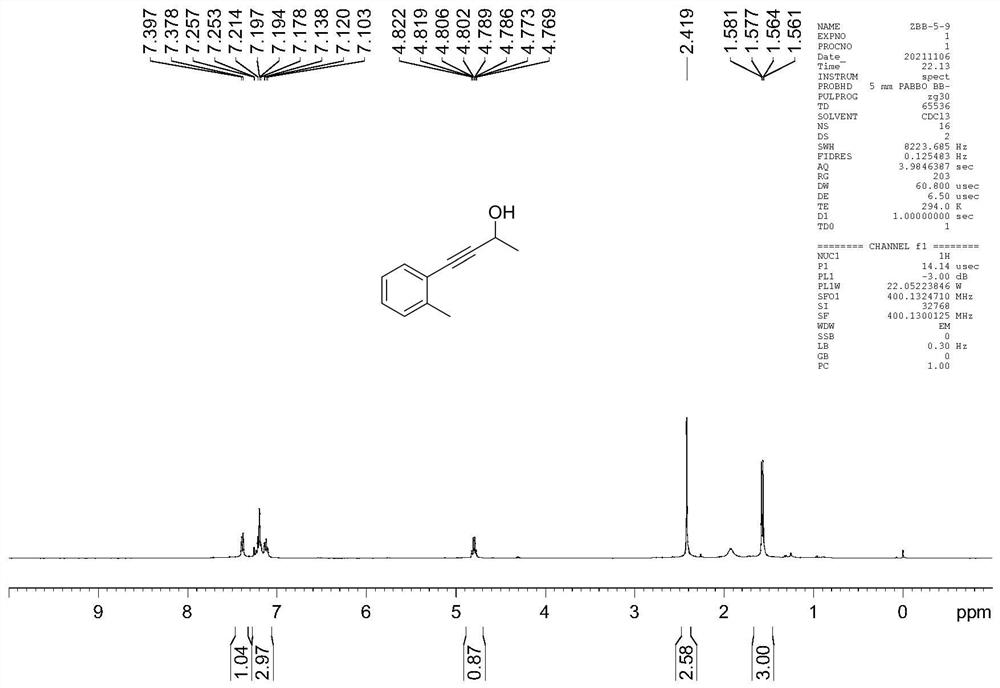
[0044] Example 2 Preparation of (2-methylphenyl)-3-butyn-2-alcohol
[0045]
[0046] In a water-free and oxygen-free glove box under argon atmosphere 4 NBr (0.02 mmol) was added to a 10 mL reaction tube, then 2 ml THF (tetrahydrofuran), then pinacol borane (1.0 mmol) was sent out of the glove box, and then (2-methylphenyl)-3 -butyn-2-one (0.2 mmol). Reaction in 60°C oil bath, TLC monitoring and I 2 The reaction was detected by color development. After the reaction was completed, it was quenched by adding sodium hydroxide solution (15mol / L), extracted and concentrated, and carried out silica gel column chromatography with petroleum ether / ethyl acetate as eluent to obtain (2-methylbenzene base)-3-butyn-2-ol. Yellow oily liquid, yield 80%. 1 H NMR (400MHz, CDCI 3) 7.40-7.38 (d, 1H), 7.26-7.10 (m, 3H), 4.82-4.77 (m, 1H), 2.42 (s, 3H), 1.58-1.56 (d, 3H).
Embodiment 3
[0047] Example 3 Preparation of 4-(2-fluorophenyl)-3-butyn-2-ol
[0048]
[0049] In an anhydrous and oxygen-free glove box under argon atmosphere 4 NBr (0.02 mmol) was added to a 10 mL reaction tube, then 2 ml THF (tetrahydrofuran), then pinacol borane (1.0 mmol) was sent out of the glove box, and then 4-(2-fluorophenyl)- 3-Butyn-2-one (0.2 mmol). Reaction in 60°C oil bath, TLC monitoring and I 2 The reaction was detected by color development. After the reaction was completed, it was quenched by adding sodium hydroxide solution (15mol / L), extracted and concentrated, and carried out silica gel column chromatography with petroleum ether / ethyl acetate as eluent to obtain 4-(2-fluoro Phenyl)-3-butyn-2-ol. Yellow oily liquid, yield 55%. 1H NMR(400MHz, CDCI3)δ7.44-7.39(m,1H), 7.33-7.26(m,1H), 7.11-7.04(m,2H), 4.82-4.77(m,1H), 1.58-1.57(d ,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com