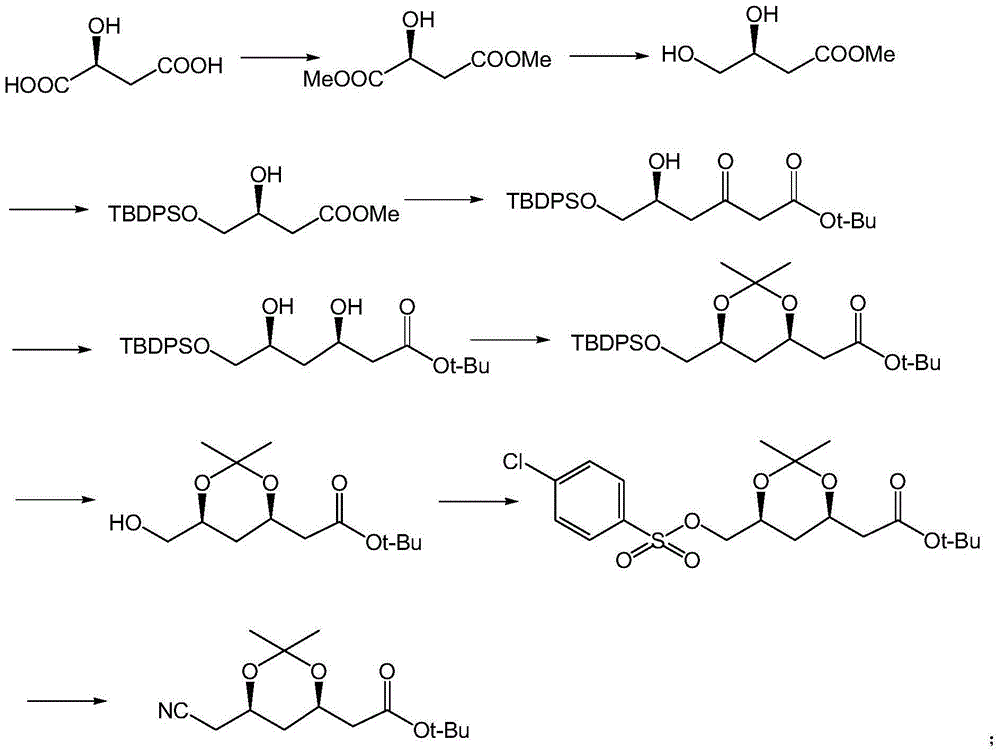
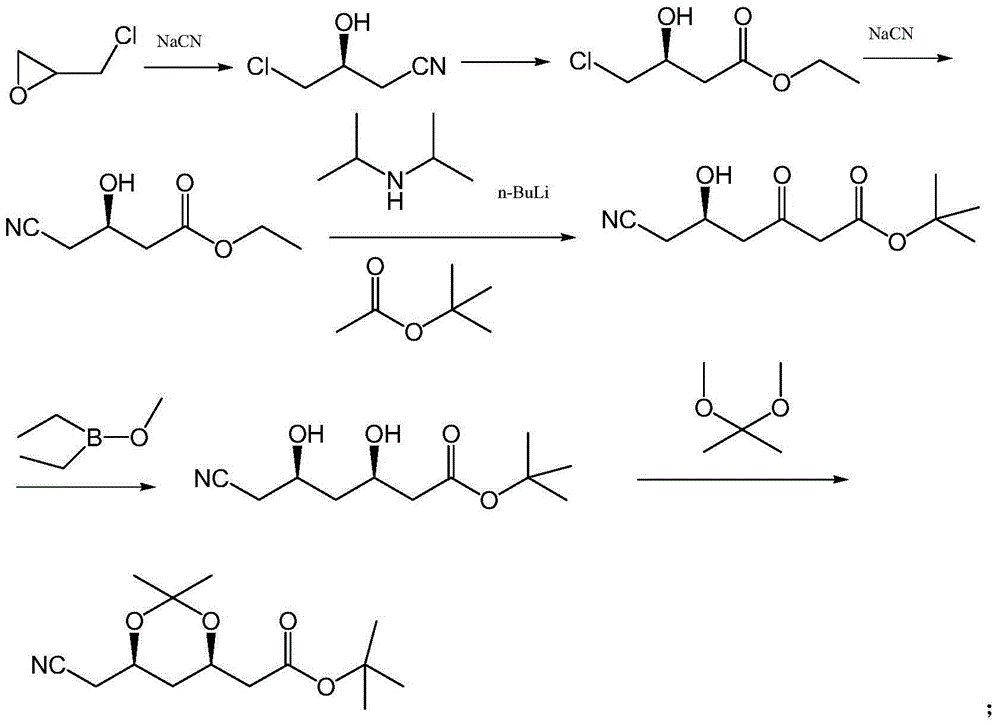
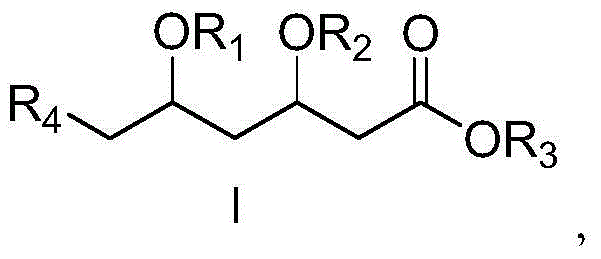
Preparation method of 3,5,6-substitued caproate derivative
A technology of hexanoate and derivatives, which is applied in the field of preparation of 3,5,6-substituted hexanoate derivatives, can solve the problems of unsuitability for industrialized large-scale production, high requirements on reaction conditions, pollution, etc., and is suitable for large-scale production. Large-scale production, cheap and easy-to-obtain reagents, and environment-friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] a) Dissolve 10 g of the lactone represented by general formula II in 50 mL of absolute ethanol, add 2 drops of concentrated hydrochloric acid, react at room temperature for 2 h, and monitor the completion of the reaction by TLC. The system was adjusted to neutral with sodium bicarbonate, concentrated to remove the solvent, diluted with 100 mL of ethyl acetate, and washed once with 50 mL of saturated brine. Dry over sodium sulfate, concentrate and remove the solvent to obtain 12.1 g of a colorless oil with a yield of 94%, which is directly used in the next reaction.
[0038] b) 10 g of the product of step a) was dissolved in 50 mL of acetone, 7.8 g of sodium iodide was added, and the reaction was refluxed for 48 h, and the reaction was complete as monitored by HPLC. The solid was removed by suction filtration, and the solvent was removed by concentration to obtain 14.0 g of light yellow oil with a yield of 97%, which was directly used in the next reaction.
...
Embodiment 1-1
[0043]
[0044] Dissolve 5 g of the product of step e) (i.e. tert-butyl 2-(6-iodomethyl-2,2-dimethyl-1,3-dioxan-4-yl)acetate) in 15 mL of DMSO and add acetic acid Potassium 2.0g, heated to 60°C for 8h. Pour into water, extract with 50mL ethyl acetate, wash with 20mL saturated brine, and dry over sodium sulfate. Concentrate to dryness to obtain 3.8 g, and recrystallize from n-heptane to obtain 3.3 g of white solid, yield 82%.
[0045] The structure of the product was confirmed by H NMR spectroscopy and electrospray ionization mass spectrometry.
[0046] 1 HNMR (300MHz, CDCl 3 ):δ1.40(s,9H),1.41(s,6H),1.48-1.73(dd,2H),2.01(s,3H),2.26-2.51(d,2H),4.09-4.34(d,2H ), 4.43(m,1H), 4.39(m,1H).
[0047] MS (ESI) m / z: (M+H) = 303.1.
Embodiment 1-2
[0049]
[0050] Dissolve 5 g of the product of step e) (i.e. tert-butyl 2-(6-iodomethyl-2,2-dimethyl-1,3-dioxan-4-yl)acetate) in 15 mL of DMSO, add cyanide Sodium chloride 0.7g, heated to 50 ℃ for 16h. Pour into water, extract with 50mL ethyl acetate, wash with 20mL saturated brine, and dry over sodium sulfate. Concentrate to dryness to obtain 3.4 g, and recrystallize from n-heptane to obtain 3.1 g of off-white solid. Starting from the lactone represented by the general formula II, the overall yield is 57.9%.
[0051] The structure of the product was confirmed by H NMR spectroscopy and electrospray ionization mass spectrometry.
[0052] 1 HNMR (300MHz, CDCl 3 ):δ1.40(s,9H),1.41(s,6H),1.48-1.73(dd,2H),2.26-2.51(d,2H),2.41-2.66(d,2H),3.8(m,1H ), 4.43(m,1H).
[0053] MS (ESI) m / z: (M+H) = 270.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com