Polypeptide or derivatives thereof and application of polypeptide or derivatives thereof in preparation of medicines for preventing and treating tumors
A technology of derivatives and drugs, applied in anti-tumor drugs, drug combinations, peptides, etc., can solve the problem of low biological stability, and achieve the effects of less toxic and side effects, significant curative effect, and safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 polypeptide
[0027] For the amino acid sequence of the polypeptide CM4, refer to SEQ ID No.1 in the sequence listing. Peptide CM4 was synthesized and purified by China Peptide Biochemical Co., Ltd.
[0028] Two unnatural amino acids S-pentylalanine (S5) were introduced for solid-phase polypeptide chain synthesis. After the solid-phase polypeptide chain is synthesized, ruthenium is used as a catalyst to perform olefin metathesis reaction (RCM) cyclization to obtain the target polypeptide. Finally, the target polypeptide is cleaved from the resin for purification. The above solid-phase polypeptide chain synthesis and purification steps were completed by China Peptide Biochemical Co., Ltd. Among them, two S-pentenylalanines (S5) are inserted into the i, i+4 positions in the amino acid sequence of the polypeptide CM4, thereby obtaining modified polypeptides of different sequences (for the amino acid sequence, refer to the sequence table SEQ...
Embodiment 2
[0040] Example 2 Detection of binding ability of polypeptide and TRB3 protein by surface plasmon resonance
[0041] The surface plasmon resonance experiment was carried out in a surface plasmon resonance instrument Biacore T200, and the operation steps were carried out according to the instructions of the plasmon resonance instrument Biacore T200. Specific steps are as follows:
[0042] 1. The purified TRB3 protein (purchased from RD Company) was amino-coupled to a CM5 chip (purchased from GE Company), and the unbound protein was eluted at a flow rate of 10 μL / min, and the surface of the chip was equilibrated for 2 hours. For the specific steps of amino coupling, elution and equilibration, please refer to the relevant instructions of GE's CM5 chip.
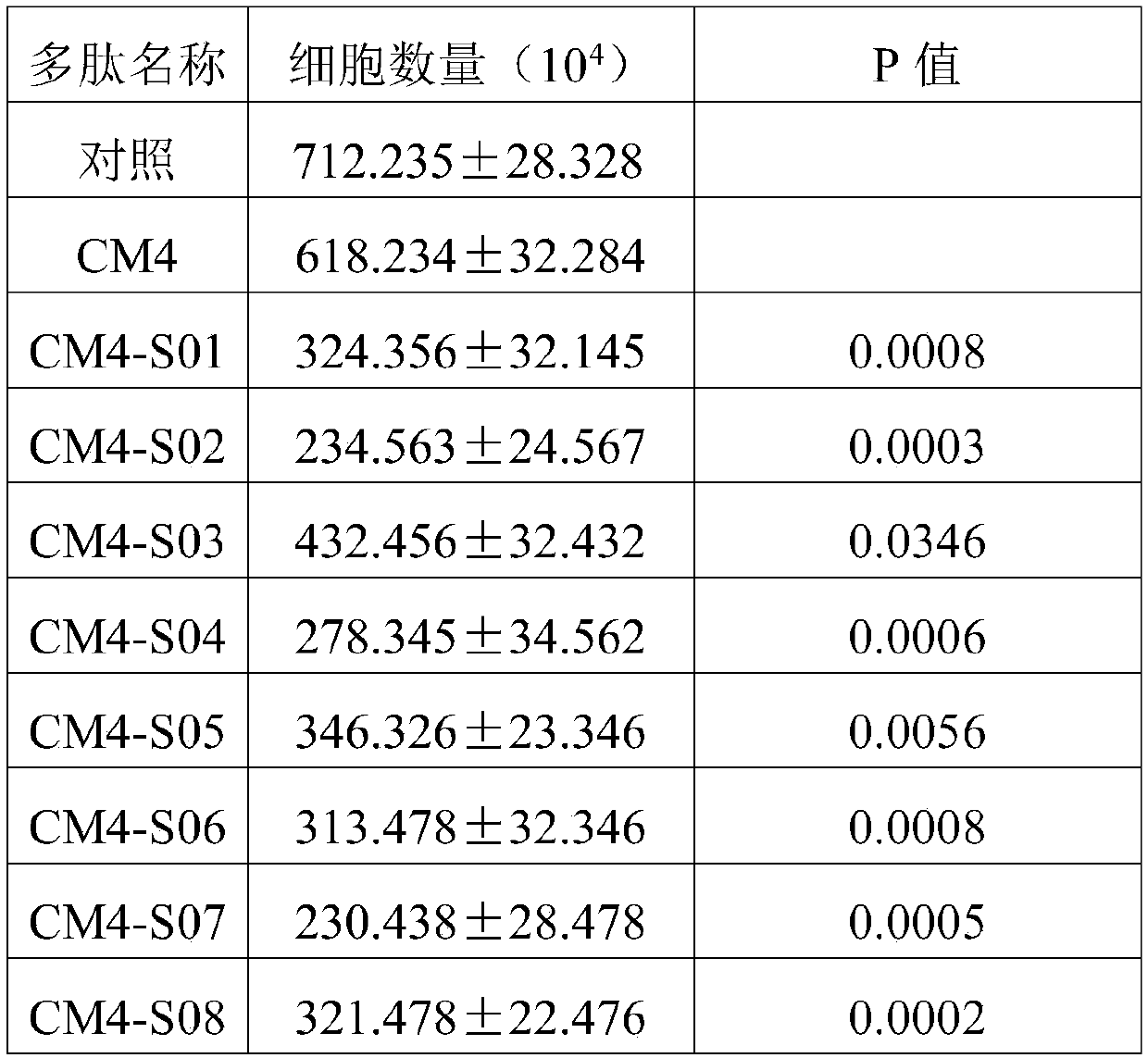
[0043] 2. Automatically inject 250 μL of CM4-S01~CM4-S10 and CM4 polypeptide fragments prepared in Example 1 with different concentrations (800, 400, 200, 50, 12.5, 6.25 and 3.125nM), and the entire surface plasmon resonance expe...
Embodiment 3
[0046] Embodiment 3 Circular dichroism method detects the alpha helical rate of polypeptide
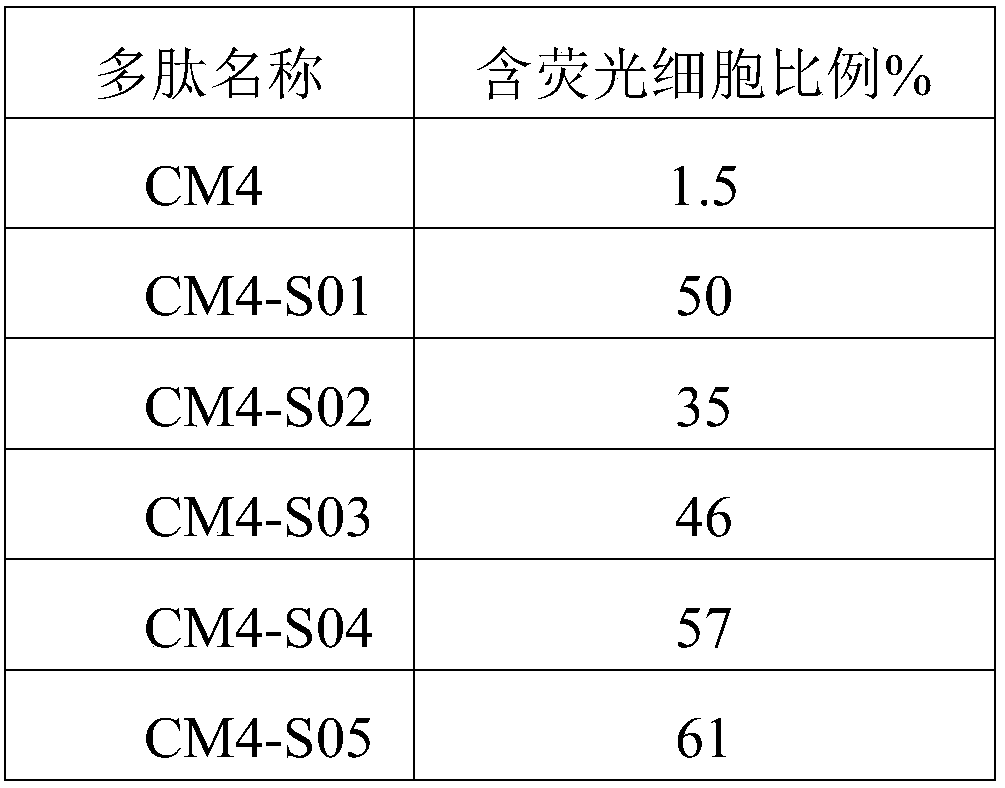
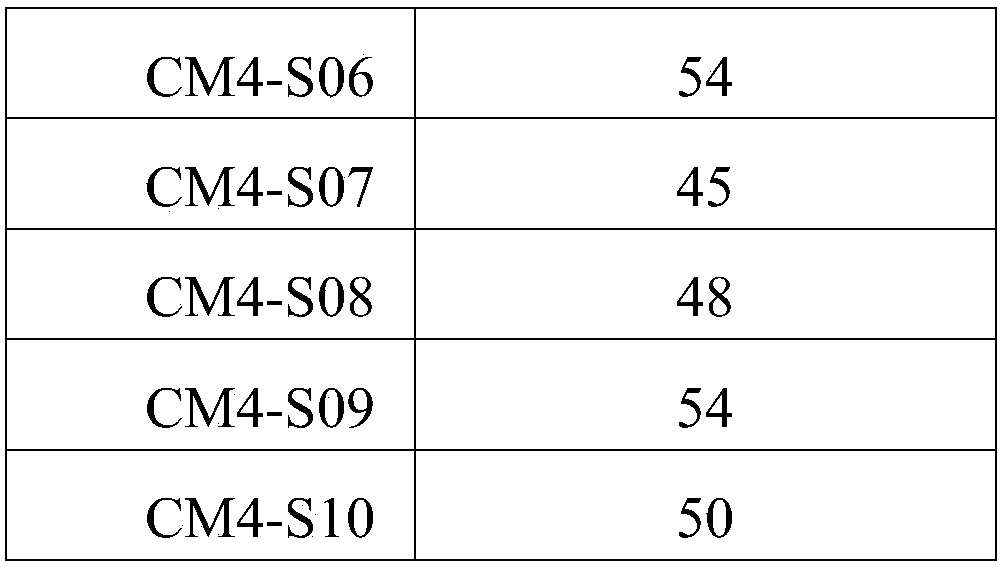
[0047] The α-helix rate of the polypeptide was detected with a circular dichroism spectrometer (purchased from Jasco, Japan). Dissolve the polypeptides CM4, CM4-S01, CM4-S02, CM4-S03, CM4-S04, CM4-S05, CM4-S06, CM4-S07, CM4-S08, CM4-S09, CM4-S10 prepared in Example 1 Into the PBS solution, the concentration on the circular dichroism analyzer was adjusted to 1 mg / mL, and the results are shown in Table 2. Table 2 shows that the α-helix rate of polypeptide CM4-S01, CM4-S02, CM4-S03, CM4-S04, CM4-S05, CM4-S06, CM4-S07, CM4-S08, CM4-S09, CM4-S10 is significantly higher in the polypeptide CM4. The maintenance of the α-helical rate of the polypeptide is an important index to increase the stability of the polypeptide. Therefore, the increase of the α-helical rate of the polypeptide CM4-S01-CM4-S10 will enhance its stability. Therefore, the increase of the α-helix rate of the polypeptide CM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com