Analysis method for determining lubiprostone test sample related substances
A lubiprostone and analytical method technology, applied in the field of drug analysis, can solve problems such as structural instability of lubiprostone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
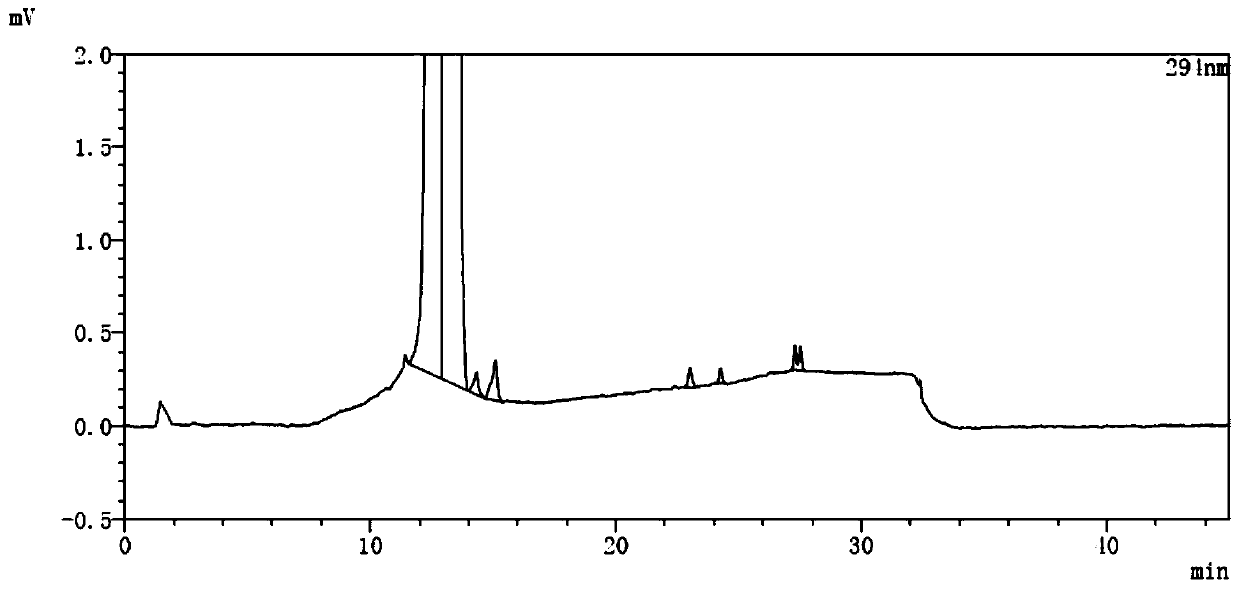
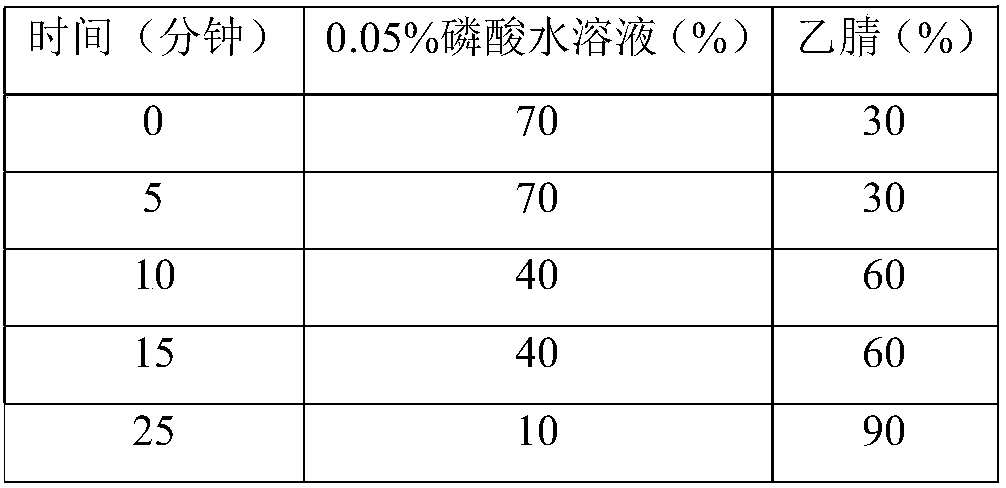
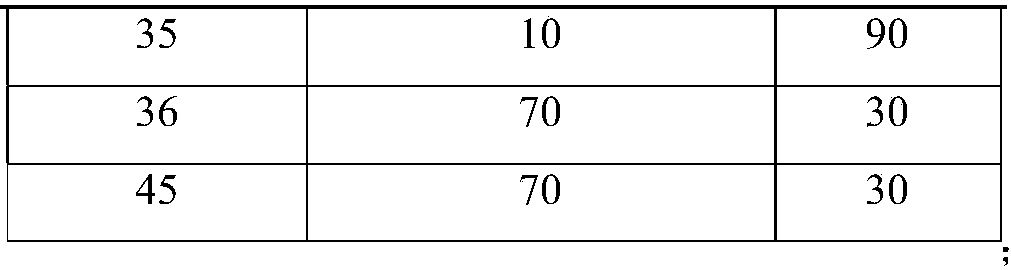
[0081] Embodiment 1 related substance determination method
[0082] Get an appropriate amount of lubiprostone test sample, accurately weighed, dissolve and dilute with acetonitrile to make a solution containing about 5 mg in every 1ml, as the test solution; get an appropriate amount of lubiprostone reference substance, accurately weighed, use Acetonitrile was dissolved and diluted to make a solution containing about 10 μg per 1 ml as a reference solution; another appropriate amount of lubiprostone, compound of formula I, compound of formula II, compound of formula III, compound of formula IV and compound of formula V , accurately weighed, dissolved with acetonitrile and diluted respectively to make a solution containing about 5 mg of lubiprostone in every 1 ml, a solution containing about 5 μg of compound of formula I in every 1 ml, a solution of about 5 μg of compound of formula II in every 1 ml, The solution containing about 5 μg of the compound of formula III in each 1 ml, ...
Embodiment 2
[0091] Embodiment 2 related substance determination repeatability experiment
[0092] Get an appropriate amount of the test sample, add acetonitrile to dissolve and dilute to make a solution containing about 5 mg per 1 ml, as the test sample solution, prepare six parts in parallel, according to the method described in Example 1, sample injection analysis, and calculate with the self-control method Each impurity and the total impurity content were used to examine the repeatability of the method. The measurement results are shown in the table below:
[0093] Table of repeatability test results
[0094] sample 1 2 3 4 5 6 Compound of formula III (%) 0.04 0.04 0.04 0.04 0.04 0.05 Formula I compound (%) not detected not detected not detected not detected not detected not detected Formula IV compound (%) not detected not detected not detected not detected not detected not detected Formula II compound (%) not detected...
Embodiment 3
[0096] Embodiment 3 related material intermediate precision experiment
[0097] According to the method described in Example 1, two analysts were respectively on two different high-performance liquid chromatographs, measured according to the related substance determination method, and counted each impurity and the total impurity content. The results of the intermediate precision of the method for determination of related substances are shown in the table below:
[0098] Table of intermediate precision results for related substances
[0099]
[0100] It can be seen from the above table that the results of the intermediate precision show that the content of the compound of formula III is 0.03% to 0.05%, the maximum single impurity content is: 0.03% to 0.04%, and the total impurity content is: 0.07% to 0.10%. The intermediate precision of this method is good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com