The preparation method of solifenacin intermediate
A solifenacin and intermediate technology, applied in the field of drug synthesis, can solve problems such as heavy metal residues and stricter requirements for heavy metal impurities, and achieve high recovery, easy-to-obtain reagents, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The invention provides a kind of preparation method of solifenacin intermediate, described preparation method comprises the following steps:
[0039] Dissolve 3-quinuclidone hydrochloride in a solvent to obtain 3-quinuclidone hydrochloride solution;
[0040] Adding a basic compound and a catalyst to the 3-quinuclidone hydrochloride solution, feeding hydrogen, stirring and reacting to obtain a reactant;
[0041] The catalyst in the reactant is recovered by filtration, and the obtained filtrate is concentrated under reduced pressure and then recrystallized;
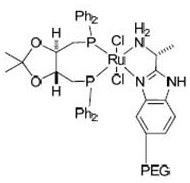
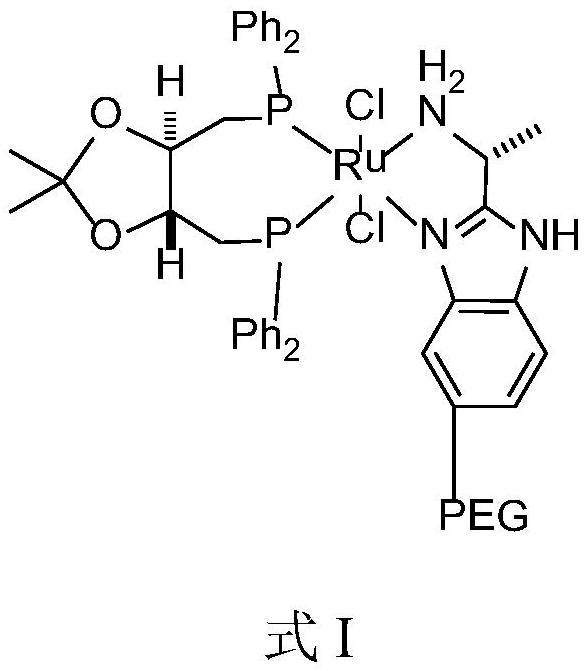
[0042] Described catalyst has the structure shown in formula I:
[0043]
[0044] The degree of polymerization of the PEG is 60-400.
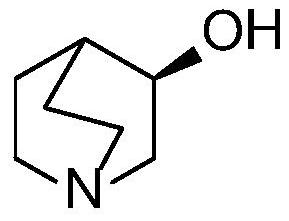
[0045] The above method adopts the asymmetric catalytic hydrogenation reduction method to prepare the solifenacin intermediate—(R)-(-)-3-quinine alcohol. In the catalyst structure, macromolecular structure polyethylene glycol (PEG) is introduced on the benzene ring of the nitrog...
Embodiment 1
[0071] This embodiment provides a preparation method of solifenacin intermediate [(R)-(-)-3-quinine alcohol], comprising the following steps:
[0072] Step 1: In a 5L autoclave, under an argon atmosphere, add 100g of 3-quinuclidinone hydrochloride from the feeding port, then add 1L of absolute ethanol to fully dissolve the raw materials, and cool down with cooling water Add 76 g of potassium tert-butoxide, stir well and continue to blow in argon gas for degassing by bubbling. The bubbling is continued for 1 hour, and the degassing is completed.
[0073] Step 2: Add 0.2 g of PEG having a structure shown in formula I through the feeding port, wherein the degree of polymerization of PEG is 180-220, and quickly close the feeding port. Replace the argon with hydrogen, slowly introduce hydrogen to 3.0Mpa, and close the inflation valve. Stir rapidly and set the temperature to 30 °C. When the pressure drops to remain constant, the reaction is considered to stop.
[0074]
[0075...
Embodiment 2
[0078] This embodiment provides a preparation method of Solifenacin intermediate [(R)-3-quinine alcohol], which is basically the same as Example 1, the difference is that the degree of polymerization of PEG in the catalyst is different, specifically comprising the following steps:
[0079] Step 1: In a 5L autoclave, under an argon atmosphere, add 100g of 3-quinuclidinone hydrochloride from the feeding port, then add 1L of absolute ethanol to fully dissolve the raw materials, and cool down with cooling water Add 76 g of potassium tert-butoxide, stir well and continue to blow in argon gas for degassing by bubbling. The bubbling is continued for 1 hour, and the degassing is completed.
[0080] Step 2: Add 0.2 g of PEG having a structure shown in formula I through the feeding port, wherein the degree of polymerization of PEG is 100-180, and quickly close the feeding port. Replace the argon with hydrogen, slowly introduce hydrogen to 3.0Mpa, and close the inflation valve. Stir rap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com