The preparation method of (r)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanol
A technology of alkoxyphenyl and alkylsulfonyl, which is applied in the field of preparation of -1--2-ethanol, can solve the problems of inability to meet drug quality standards, heavy metals and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
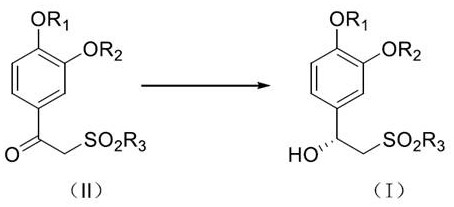
[0047] The preparation method of (R)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanol according to one embodiment of the present invention comprises the following steps:
[0048] S101: providing a compound of the structure shown in formula (II);
[0049]
[0050] Among them, R 1 , R 2 and R 3 each independently for C 1-16 Alkyl, 3-8 membered cycloalkyl, 5-6 membered aryl or 5-6 membered heteroaryl; further, R 1 , R 2 and R 3 each independently for C 1-6 Alkyl, 3-6 membered cycloalkyl, 6 membered aryl or 6 membered heteroaryl; further, R 1 , R 2 and R 3 each independently for C 1-4 Alkyl or phenyl.
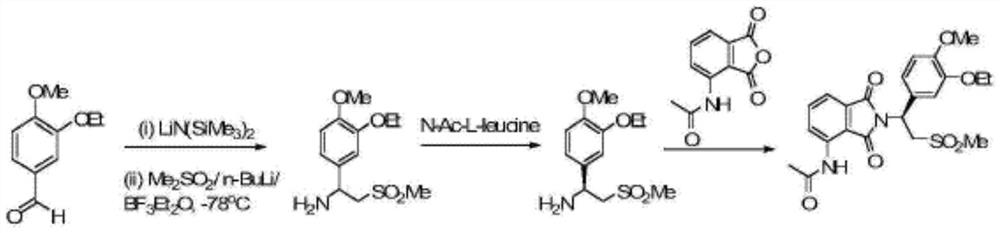
[0051] The structural compound represented by formula (II) can be obtained by using commercially available raw materials, or can be synthesized by existing methods, and is not particularly limited here. For example: Synthesize using the following method:
[0052]
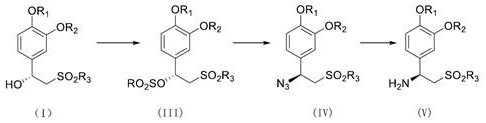
[0053] Remove the ketone carbonyl α-position active wave hydrogen of the compound shown in th...
Embodiment 1
[0103]
[0104] In a 5L autoclave, under an argon atmosphere, add 100g of compound 1 from the feeding port, then add 1.5L of toluene to fully dissolve the raw material, stir fully and continuously pass in argon to degas by bubbling, and continue bubbling for 1h. Degassing is complete. Add 0.2g catalyst ( Where n is 12), quickly close the feeding port. Replace the argon with hydrogen, slowly introduce hydrogen to 3.0Mpa, and close the inflation valve. Rapidly stirred reactions were carried out at 25-40°C. When the pressure drops to remain constant, the reaction is considered to stop. Sampling was sent for liquid phase analysis to confirm the conversion rate. After the reaction, the system was filtered with suction. The catalyst was removed, and the filtrate was concentrated under reduced pressure to obtain an off-white solid, which was tested for residual heavy metals, chiral purity, catalyst recovery, and absolute configuration.
Embodiment 2
[0106]
[0107] In a 5L autoclave, under an argon atmosphere, add 100g of compound 1 from the feeding port, then add 1.5L of toluene to fully dissolve the raw material, stir fully and continuously pass in argon to degas by bubbling, and continue bubbling for 1h. Degassing is complete. Add 0.2g catalyst ( Where n is 16), quickly close the feeding port. Replace the argon with hydrogen, slowly introduce hydrogen to 3.0Mpa, and close the inflation valve. Rapidly stirred reactions were carried out at 25-40°C. When the pressure drops to remain constant, the reaction is considered to stop. Sampling was sent for liquid phase analysis to confirm the conversion rate. After the reaction, the system was filtered with suction. The catalyst was removed, and the filtrate was concentrated under reduced pressure to obtain an off-white solid, which was tested for residual heavy metals, chiral purity, catalyst recovery, and absolute configuration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com