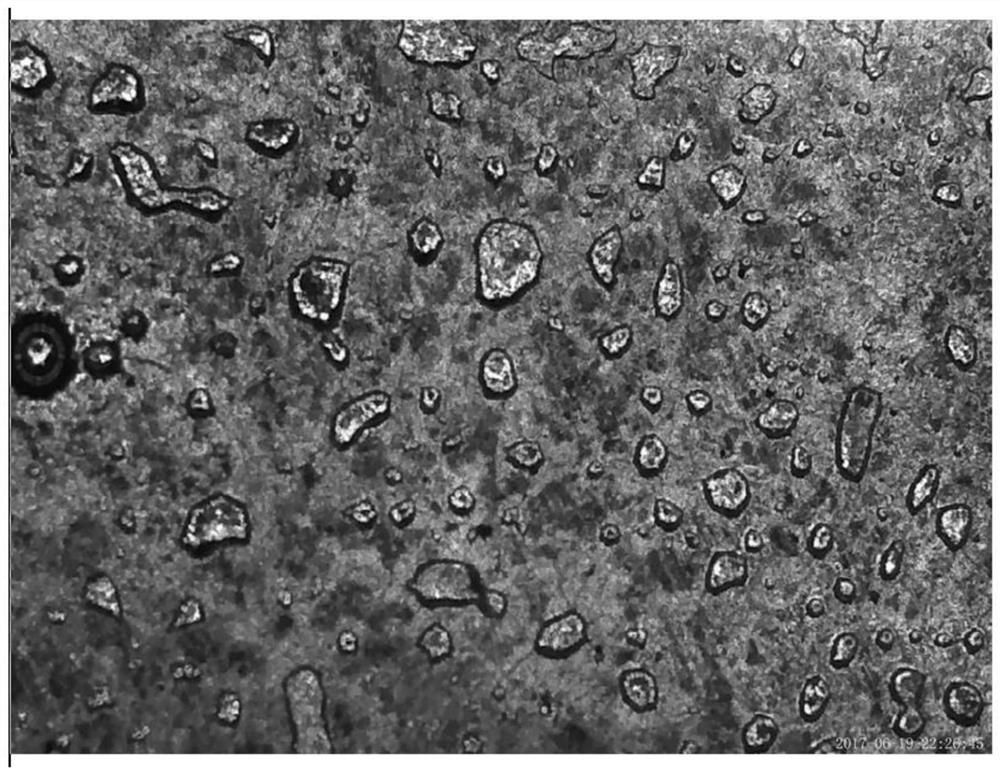
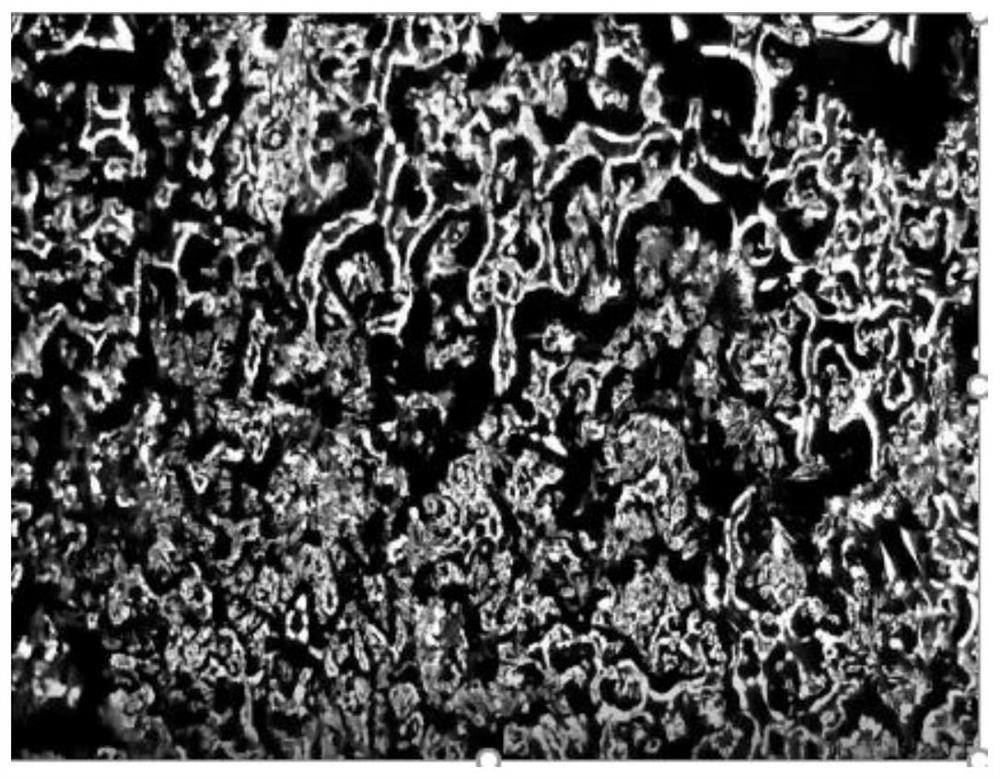
Dendrite-free alkali metal ion battery based on negative electrode surface solid/liquid phase conversion
A technology of alkali metal ion and liquid phase conversion, applied in the field of alkali metal ion batteries, can solve the problems of poor cycle performance of lithium metal negative electrodes, contact between positive and negative electrodes, electrolyte decomposition, etc., achieve high capacity, improve charge and discharge capacity, and high Effect of Energy Density and Tap Density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0044] A dendrite-free alkali metal ion battery based on the solid / liquid phase conversion on the surface of the negative electrode. The sodium metal sheet is used as the negative electrode of the battery, the potassium metal sheet is used as the positive electrode of the battery, and the electrolyte is 0.8mol / L KPF 6 Dissolved in ethylene carbonate (EC) diethyl carbonate: diethyl carbonate (DEC): fluoroethylene carbonate (FEC) in a solvent with a mass ratio of 4:4:2 to prepare a simulated battery.
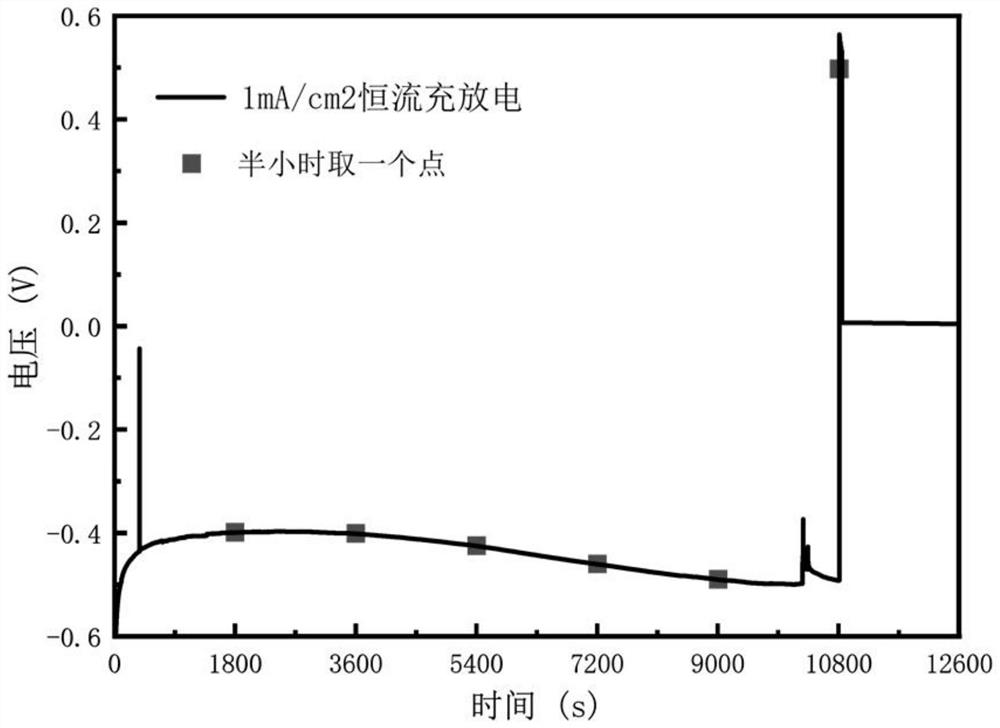
[0045] A dendrite-free alkali metal ion battery based on the solid / liquid phase transformation on the surface of the negative electrode described in this embodiment, the prepared simulated battery uses an electrochemical workstation, and adopts an electrochemical method of constant current charge and discharge, firstly conducts a 1mA / cm 2 The current density is charged, and the charging time is 3h.
[0046] A dendrite-free alkali metal ion battery based on the solid / liquid phase t...
specific Embodiment approach 2
[0052] A dendrite-free alkali metal ion battery based on the solid / liquid phase transformation on the surface of the negative electrode, the material of the positive electrode sheet is a positive electrode material containing potassium ions. The detailed preparation method: prepare 25ml 0.4mol / L anhydrous potassium ferrocyanide For liquid A, prepare 25ml of 0.4mol / L anhydrous ferric chloride as liquid B, prepare 150ml of 0.5mol / L potassium chloride as liquid C, add liquid A and liquid B dropwise into liquid C, and keep stirring to produce rapidly precipitation. Aged at room temperature for 24 hours, centrifuged and washed three times with deionized water and absolute ethanol respectively, and dried in vacuum at 80° C. for 24 hours to prepare the Prussian blue potassium active material.
[0053] A kind of dendrite-free alkali metal ion battery based on negative electrode surface solid / liquid phase conversion described in this embodiment, weigh 0.4g of Prussian blue potassium ac...
specific Embodiment approach 3
[0057] A dendrite-free alkali metal ion battery based on the solid / liquid phase transformation on the surface of the negative electrode, the material of the positive electrode sheet is a positive electrode material containing potassium ions. The detailed preparation method: prepare 160ml 1mmol anhydrous potassium ferrocyanide as A liquid , prepare 40ml2mmol anhydrous ferric chloride as B liquid, add B liquid into A liquid dropwise, and keep stirring, and precipitate rapidly. Aged at room temperature for 24 hours, centrifuged and washed three times with deionized water and absolute ethanol respectively, and dried in vacuum at 80° C. for 24 hours to prepare the Prussian blue potassium active material.
[0058] A dendrite-free alkali metal ion battery based on the solid / liquid phase transformation on the surface of the negative electrode described in this embodiment, weighs 0.4g of Prussian blue potassium active material, 0.05g of conductive carbon and 0.05g of PVDF, and paste, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com