Beta hairpin antimicrobial peptide with cross-chain interaction between tryptophan and histidine and preparation method thereof
A technology of histidine cross-chain and interaction, applied in the field of beta hairpin antimicrobial peptides and preparation, can solve the problems of shortened sequence length, high synthesis cost, difficult preparation, etc., and achieves strong inhibitory effect, simple synthesis method, and short sequence length. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Design of Antimicrobial Peptides
[0016] The amino acid sequence of the antimicrobial peptide WHFPG is:
[0017] Arg Trp Phe His Phe Pro Gly Arg Trp Phe His Phe-NH 2
1 5 10 12
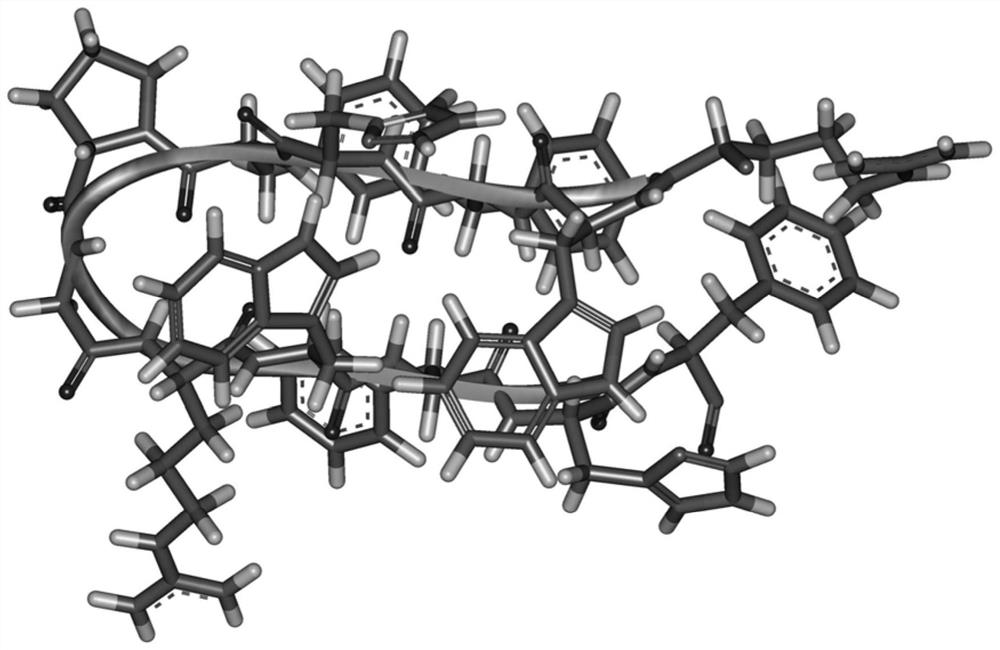
[0018] Using the arrangement principle of the β hairpin structure, the interaction of tryptophan and histidine forms the mutual attraction of two β side chains, and the PG corner is used as the central corner unit to design a cross-chain interaction containing tryptophan and histidine Antimicrobial peptide template XWYHYPGXWYHY-NH 2 , wherein X is a positively charged amino acid, Y is a hydrophobic amino acid, when X=R, Y=F, the antimicrobial peptide is named WHFPG. The sequences of the antimicrobial peptides are shown in Table 1.
[0019] Table 1 Amino Acid Sequence of Derived Peptides
[0020]
[0021] The sequence length of WHFPG is 12, two tryptophans and four phenylalanines provide sufficient hydrophobicity, and the corner unit is a PG unit. Containing two arginines and two hist...
Embodiment 2
[0023] Synthesis of WHFPG Antimicrobial Peptide by Solid Phase Chemical Synthesis
[0024] 1. The preparation of antimicrobial peptides is carried out one by one from the C-terminal to the N-terminal, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed chain protection resin;
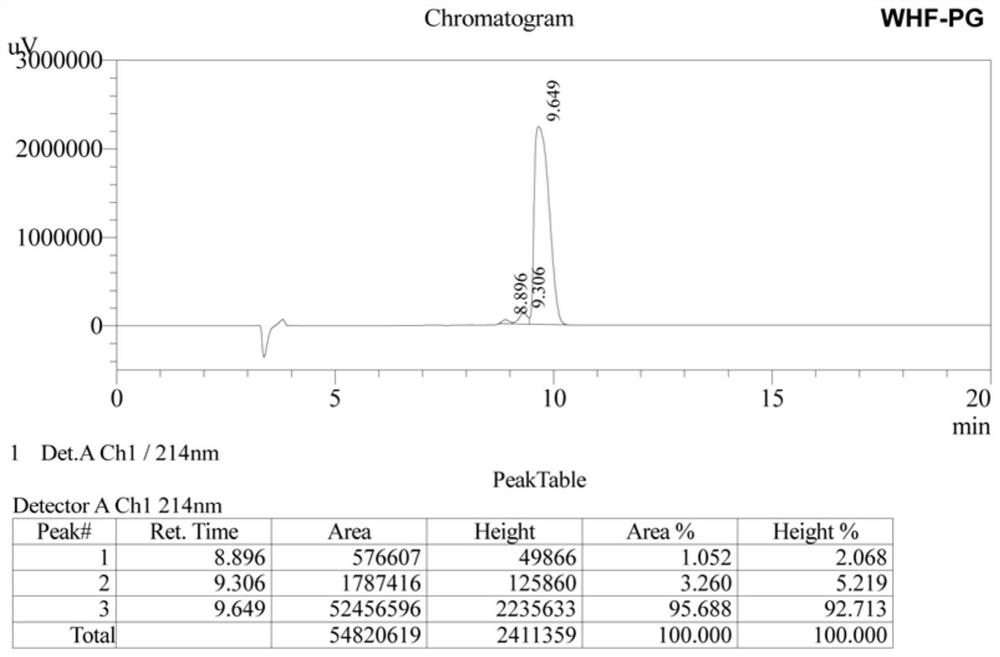
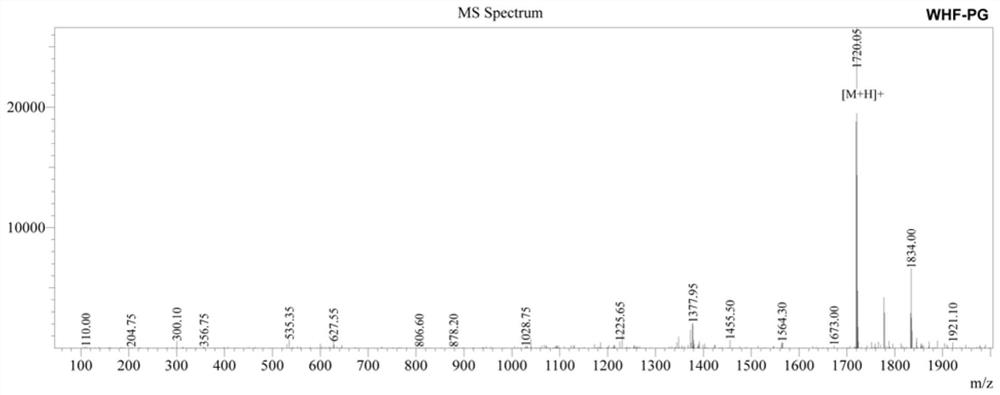
[0025] 2. Add a cleavage reagent to the peptide resin obtained above, react for 2 hours at 20°C in the dark, filter; wash the precipitate with TFA (trifluoroacetic acid), mix the washing liquid with the above filtrate, concentrate with a rotary evaporator, and t...
Embodiment 3
[0028] Embodiment 3: the mensuration of antimicrobial peptide antibacterial activity
[0029] 1. Determination of antibacterial activity: the determination method adopts the micro broth dilution method. Bacterial strains were incubated overnight at 37 °C with constant shaking at 220 rpm and then transferred to new MHB until log phase of growth. The peptide solution was added to 0.2% bovine serum albumin containing 0.01% acetic acid and serially diluted. 50 μL of bacteria Incubate with 50 μL of bovine serum albumin (pH=7.4 or 6.0) containing different concentrations of peptides at 37° C. for 18-24 hours. Each test was replicated 3 times, and each replicate had 2 parallels. MHB with and without bacteria were used as positive and negative controls, respectively. By measuring the OD at 492 nm, the MIC was calculated as the lowest concentration of the peptide for which no microbial growth was observed both visually and spectrophotometrically. The test results are shown in Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com