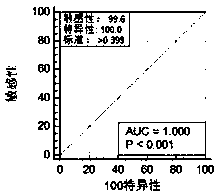
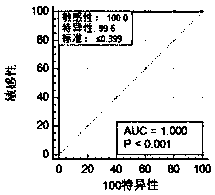
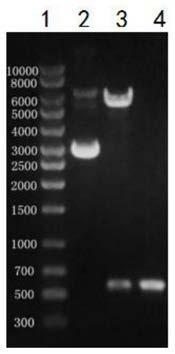
Kit for screening and detecting African swine fever virus P30 protein monoclonal antibody and preparation method thereof
An African swine fever virus, P30 technology, applied in virus disease diagnosis technology, animal quarantine, and biomedicine fields, can solve the problem of lack of methods and kits, and achieve the effect of improving screening efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 African swine fever virus P30 protein
[0027] 1 material
[0028] 1.1 Strains and vectors Competent cells of Escherichia coli BL21 were purchased from Bomed Technology Co., Ltd.; gene synthesis was synthesized by General Biosystems (Anhui) Co., Ltd. (providing plasmid PET-30a).
[0029]1.2 The main reagent DNAMarker was purchased from Thermo Company, and the plasmid mini-extraction kit was purchased from AXYGEN Company; other chemical reagents were domestic analytical grade.
[0030] 1.3 Instruments Centrifμge 5804R desktop high-speed centrifuge was purchased from Eppendorf, Germany; PCR instrument, nucleic acid electrophoresis instrument, VerSaDoc2000 gel imaging system were purchased from Bio-Rad, USA; HZQ-C air bath constant temperature double-layer oscillator was purchased from Harbin Dongming Medical Instrument factory; SW-CJ-IF ultra-clean bench, Antai Company of Sujing Group; -80℃ refrigerator, Sanyo, Japan.
[0031] 1.4 Prepara...
Embodiment 2
[0044] Embodiment 2 Establishment of the kit for identification of African swine fever virus P30 protein monoclonal antibody
[0045] 1 Materials and methods
[0046] 1.1 Test material
[0047] 1.1.1 The purified African swine fever virus P30 protein was prepared by Zhejiang Sci-Tech University.
[0048] 1.1.2 ELISA plate was purchased from Corning Company of the United States.
[0049] 1.1.3 Goat anti-mouse enzyme-labeled antibodies were purchased from Huamei Biological Company.
[0050] 1.1.4 The main reagents TMB single-component chromogenic solution was purchased from Beijing Suo Laibao Technology Co., Ltd.; other chemical reagents were domestic analytical grade.
[0051] 1.1.5 Freund's complete adjuvant and Freund's incomplete adjuvant were purchased from Sigma.
[0052] 1.1.6 BALB / c mice were purchased from Yangzhou University.
[0053] 1.2 Test method
[0054] 1.2.1 Preparation and testing of positive serum and negative serum
[0055] 1.2.1.1 Preparation of negat...
Embodiment 3
[0106] The detection method of embodiment 3 African swine fever virus P30 protein monoclonal antibody identification kit
[0107] 1. Sample dilution: use the sample diluent to dilute the sample to be tested 1:100 times;
[0108] 2. Add samples: Add diluted samples, negative control and positive control, among which, positive control and negative control are repeated twice, 100 μl / well, incubated at 37°C for 1 hour, washed 3 times with 1×PBST, 300 μl per well , 3 minutes each time, and pat dry for the last time;
[0109] 3. Add goat anti-mouse enzyme-labeled antibody: add goat anti-mouse enzyme-labeled antibody to each reaction well, 100 μl / well, incubate at 37°C for 1 hour, wash 5 times with 1×PBST, 300 μl per well, 3 minutes each time, and finally Pat dry once;
[0110] 4. Add chromogenic solution: add 100 μl chromogenic solution to each reaction well, and develop color at 37°C in the dark for 10 minutes;
[0111] 5. Add stop solution: add 50 μl stop solution to each react...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap