Lipocalin-type prostaglandin d2 synthase production accelerating agent
A technology of lipocalin and prostaglandin, which can be used in drug combination, biological testing, biological material analysis, etc., and can solve problems such as unknown promotion effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Embodiment 1 The manufacture of this extract
[0103]The vaccinia virus was inoculated intradermally in the skin of healthy mature rabbits, and the pockmarked skin was excised and harvested. After cleaning and disinfecting the collected skin with phenol solution, remove the excess phenol solution, crush it, add the phenol solution to mix, let it stand for 3-7 days, and then stir for 3-4 days while heating to 35-40°C. Then, the extract liquid obtained by solid-liquid separation was adjusted to pH 4.5-5.2 with hydrochloric acid, and after heat treatment at 90-100° C. for 30 minutes, protein was removed by filtration. Then the filtrate was adjusted to pH 9.0-9.5 with sodium hydroxide, heat-treated at 90-100° C. for 15 minutes, and then solid-liquid separation was performed.
[0104] The obtained protein-removing solution was adjusted to pH 4.0-4.3 with hydrochloric acid, 2% of the mass of the protein-removing solution was added with activated carbon, stirred for 2 hours, ...
Embodiment 2
[0105] Embodiment 2 pharmacological test
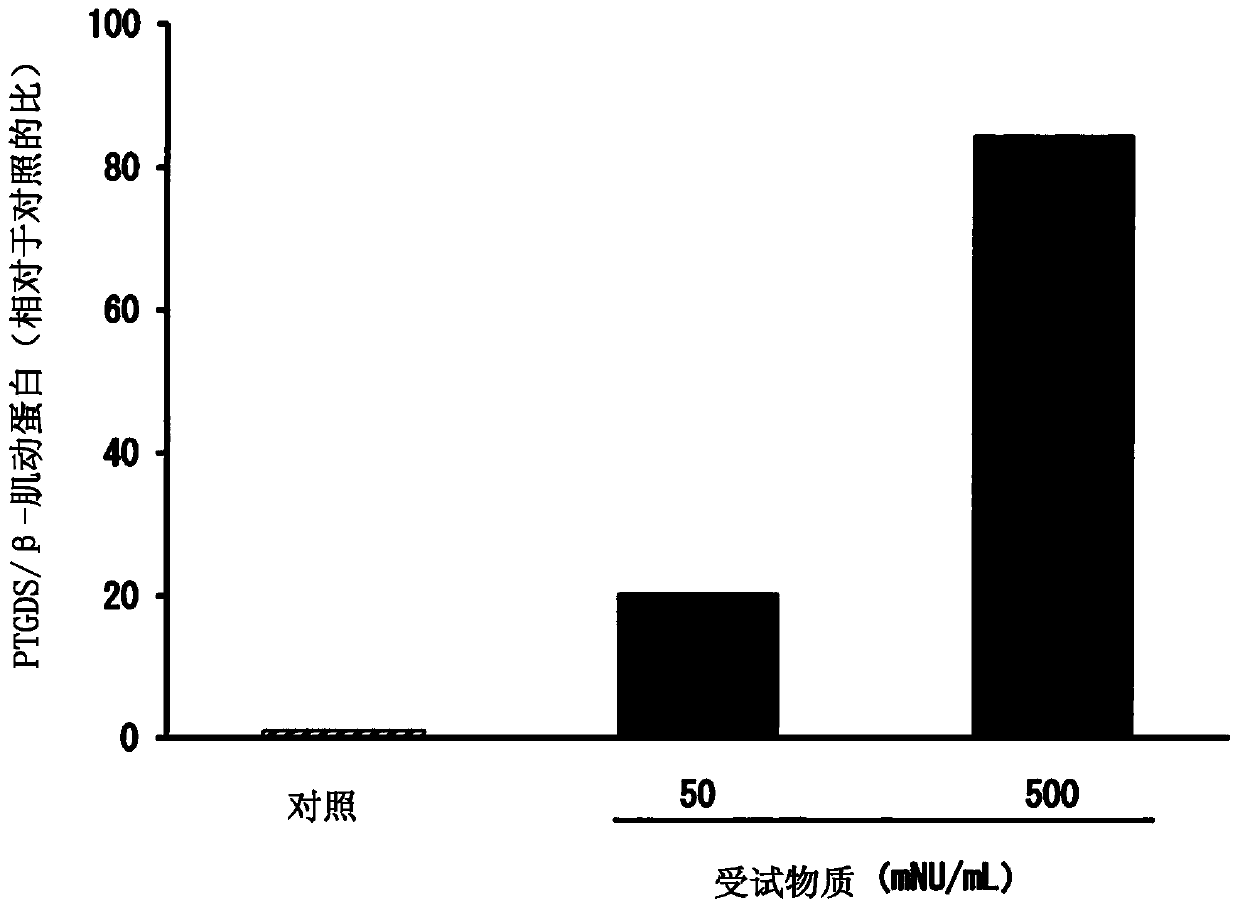
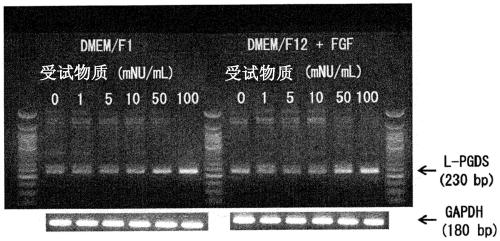
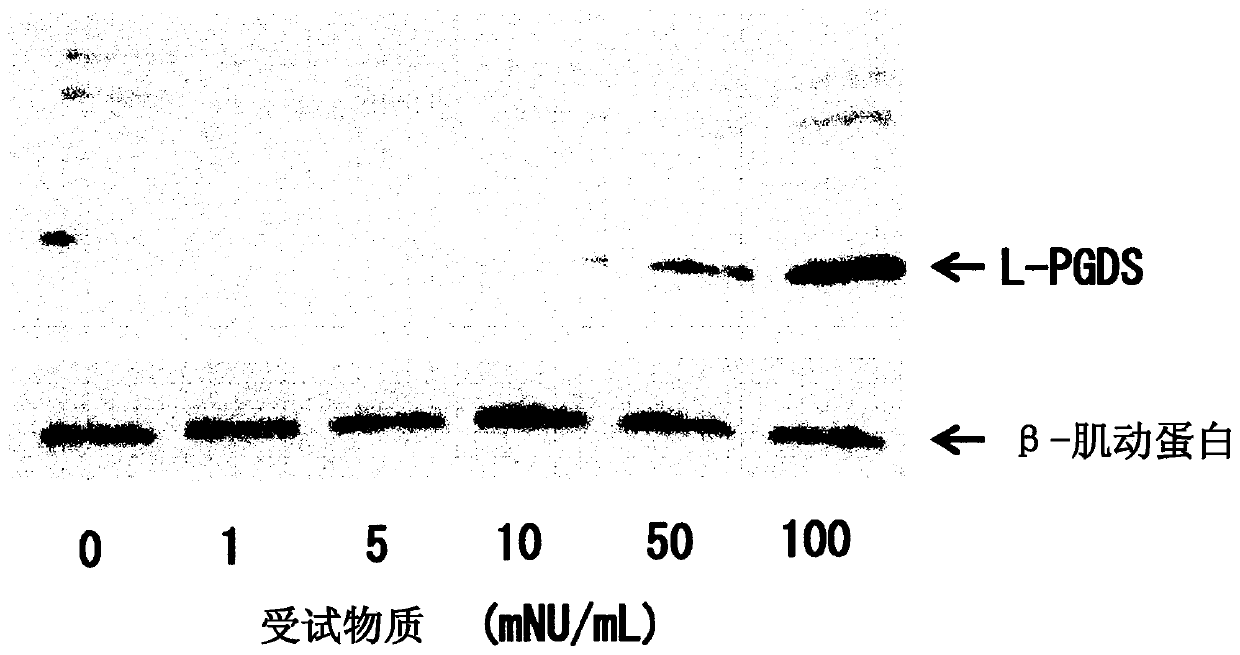
[0106] Next, the method and results of the pharmacological test on the L-PGDS production-promoting effect using the present extract obtained in Example 1 above as the test substance are shown. In addition, in the following pharmacological test, regarding the introduction of cerebral infarction into C.B-17 mice and the isolation and culture of iSCs from the cerebral infarction focus, according to Nakagomi, T. et al. Eur. J. Neurosci., 29, 1842_1852 , carried out by the method described in 2009.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com