General type DNA vaccine for influenza A, and preparation method and application of general type DNA vaccine for influenza A
A DNA vaccine, influenza A technology, applied in the fields of biopharmaceuticals and genetic engineering, to achieve the effect of easy storage and transportation, high safety and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
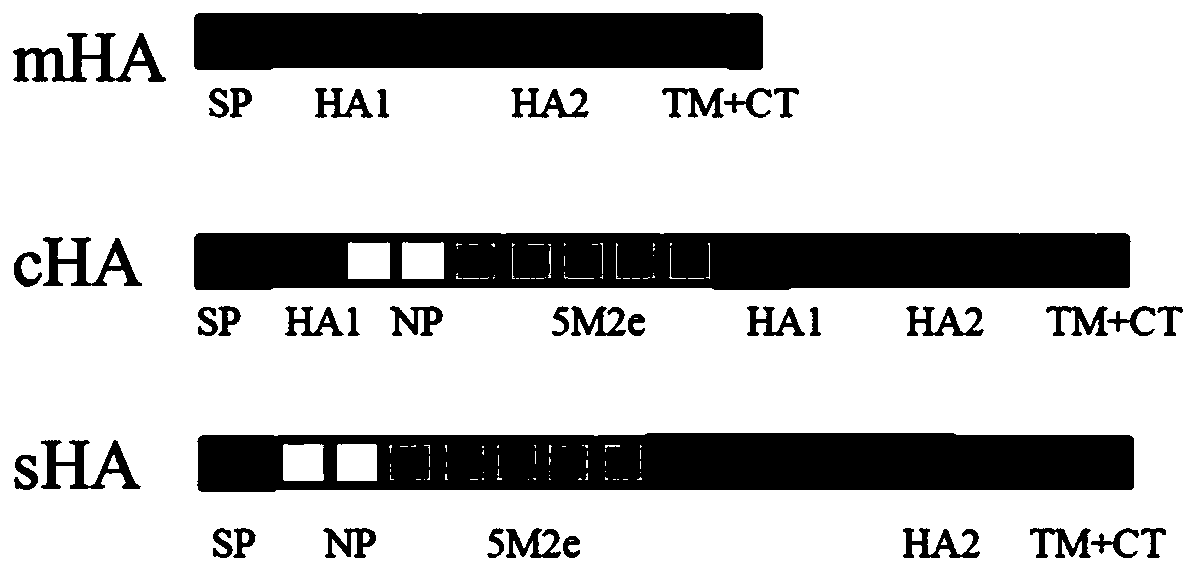
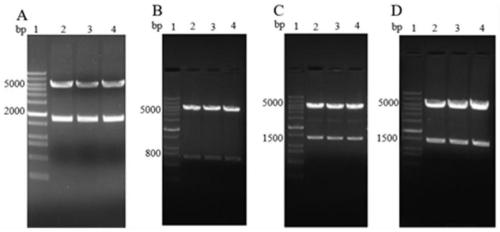
[0044] Example 1 Preparation and Identification of 3 Kinds of Recombinant Plasmids
[0045] 1. Materials
[0046] Main reagents: E.coli XL10-Gold competent cells were prepared and preserved by our laboratory, gene synthesis was provided by Jinweizhi Company, technical services such as recombinant plasmid gene sequencing and primer synthesis were provided by Jinweizhi Company. Endonucleases were purchased from Thermo Company, antibiotics, etc. were purchased from Suleibao Company, HA rabbit polyclonal antibody was purchased from Beijing Yiqiao Sino Biological Company, M2 mouse monoclonal antibody, and NP mouse monoclonal antibody were purchased from abcam Company.
[0047] 2. Method
[0048] 2.1 Extract RNA from H5N1 virus and then reverse transcribe it into cDNA, use the primers of HAF and HAR of H5N1 respectively with cDNA as template, SEQ ID NO.9, SEQ ID NO.10 are the amplification primers of HA fragment, carry out PCR amplification Increase (94°C for 5 minutes, 94°C for 3...
Embodiment 2
[0059] Example 2 Influenza A Universal DNA Vaccine Experimental Immunization Research
[0060] 1. Materials
[0061] HPR-labeled goat anti-mouse IgG, IgG1, and IgG2a were purchased from Southern Biotechnology, ELISApot detection kits for mouse IFN-γ and IL-4 were purchased from Mabtech, and TMB was purchased from Sigma. BALB / c female mice aged 6-8 weeks were purchased from Beijing Weitong Lihua Company.
[0062] 2. Method
[0063] 2.1 Immunity and attack
[0064] For immune challenge design, 180 healthy female Balb / c mice were randomly divided into 6 immunization groups, 30 mice in each group, and the experimental groups were immunized with pcDNA 3.1-HA+adjuvant (CpG+MPLA), pcDNA3.1 -mHA+adjuvant (CpG+MPLA), pcDNA 3.1-cHA+adjuvant (CpG+MPLA), pcDNA3.1-sHA+adjuvant (CpG+MPLA), control group immune pcDNA 3.1+adjuvant group (CpG+MPLA), The blank group is the PBS group. A total of 3 immunizations were performed, all of which were injected into the tibialis anterior muscle of ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap