HPLC analysis method of n-(phenylsulfonyl)benzamide compounds
An analytical method and technology of substances, applied in the direction of analytical materials, material separation, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
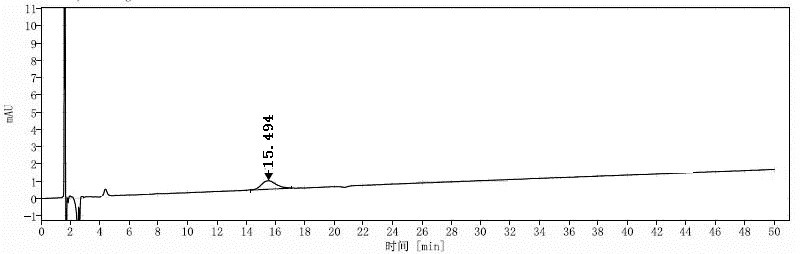
[0099] Embodiment 1: the mensuration of formula I compound chiral purity
[0100] High performance liquid chromatography conditions:
[0101] Chromatographic column: Daicel Chiralpak IC (250 mm×4.6 mm, 5.0 µm).
[0102] Mobile phase volume ratio: n-hexane / isopropanol / dichloromethane / tetrahydrofuran / acetic acid / diethylamine=700 / 70 / 80 / 150 / 1 / 0.2.
[0103] Detection wavelength: 282nm.
[0104] Flow rate: 2.0ml / min.
[0105] The column temperature was 33°C.
[0106] Injection volume: 20 μl.
[0107] Diluent: The volume ratio of dichloromethane to mobile phase is 1:1.
[0108] Experimental procedure
[0109] Solution preparation: Take about 20 mg of the compound of formula I, accurately weigh it and place it in a 10 ml measuring bottle, add 5 ml of dichloromethane, after the sample is completely dissolved, dilute to the mark with mobile phase, shake well, and use it as the test solution I;
[0110] Take 1 tablet of the compound of formula I and grind it into fine powder, tran...
Embodiment 2
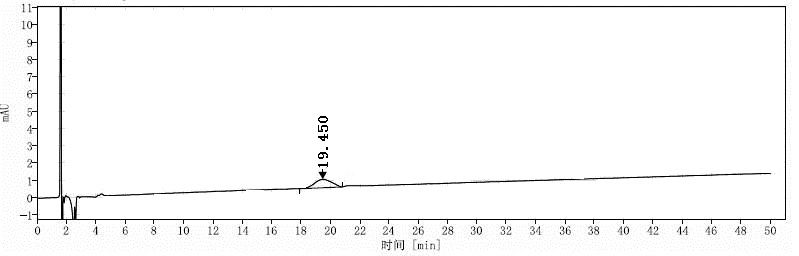
[0119] Embodiment 2: the mensuration of formula I compound chiral purity
[0120] High performance liquid chromatography conditions:
[0121] Chromatographic column: Daicel Chiralpak IC (250 mm×4.6 mm, 5.0 µm).
[0122] Mobile phase volume ratio: diethylamine / triethylamine / tetrahydrofuran / ethanol / dichloromethane / n-hexane=1 / 1 / 120 / 130 / 50 / 700.
[0123] Detection wavelength: 287nm.
[0124] Flow rate: 1.0ml / min.
[0125] Column temperature: 33°C.
[0126] Injection volume: 20 μl.
[0127] Diluent: The composition is the same as that of the mobile phase.
[0128] Experimental procedure
[0129] Solution preparation: take about 20mg of the compound of formula I, accurately weigh it and place it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and use it as the test solution;
[0130] Take about 20mg of (S)-compound reference substance of formula I, accurately weigh it and place it in a 50ml measuring bottle, dissolve it with a diluent an...
Embodiment 3
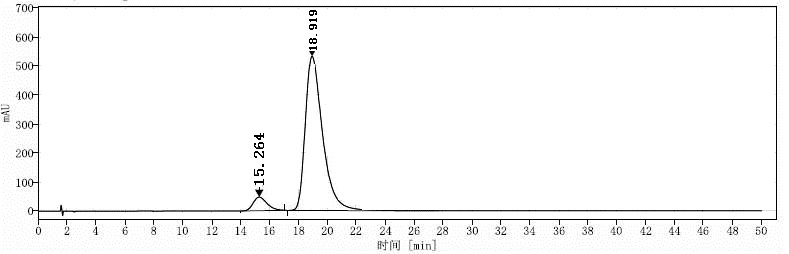
[0136] Embodiment 3: the mensuration of formula I compound chiral purity
[0137] High performance liquid chromatography conditions:
[0138] Chromatographic column: Daicel Chiralpak IC (250 mm×4.6 mm, 5.0 µm).
[0139] Mobile phase volume ratio: tetrahydrofuran / ethanol / dichloromethane / n-hexane=150 / 100 / 50 / 700.
[0140] Detection wavelength: 282nm.
[0141] Flow rate: 1.0ml / min.
[0142] Column temperature: 33°C.
[0143] Injection volume: 10 μl.
[0144] Diluent: The volume ratio of dichloromethane to mobile phase is 1:1.
[0145] Experimental procedure
[0146] Solution preparation: Take about 30 mg of the compound of formula I, accurately weigh it and place it in a 20 ml measuring bottle, dissolve it with 10 ml of dichloromethane, dilute to the mark with mobile phase, shake well, and use it as the test solution;
[0147] Take about 30mg of (S)-compound reference substance of formula I, accurately weigh it and place it in a 20ml measuring bottle, dissolve it with 10ml of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com