A kind of intelligent photosensitizer and its preparation method and application
A photosensitizer and intelligent technology, applied in the field of biochemistry, can solve the problems of the persistent photosensitivity of the residual photosensitizer, and achieve the effects of good singlet oxygen generation ability, good application prospect and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
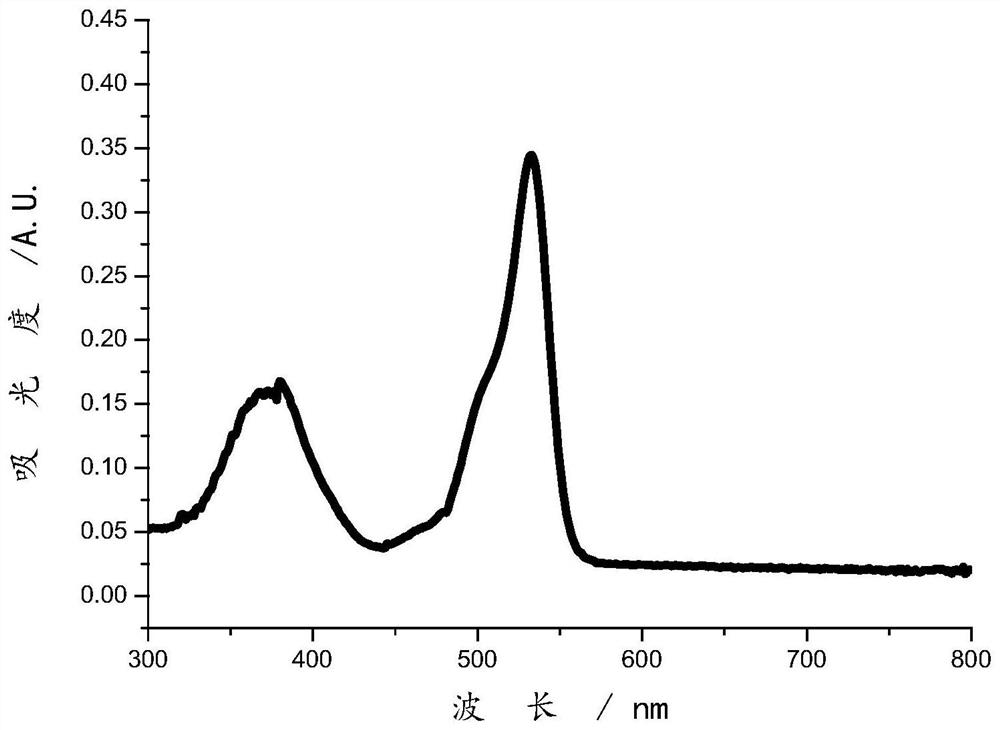
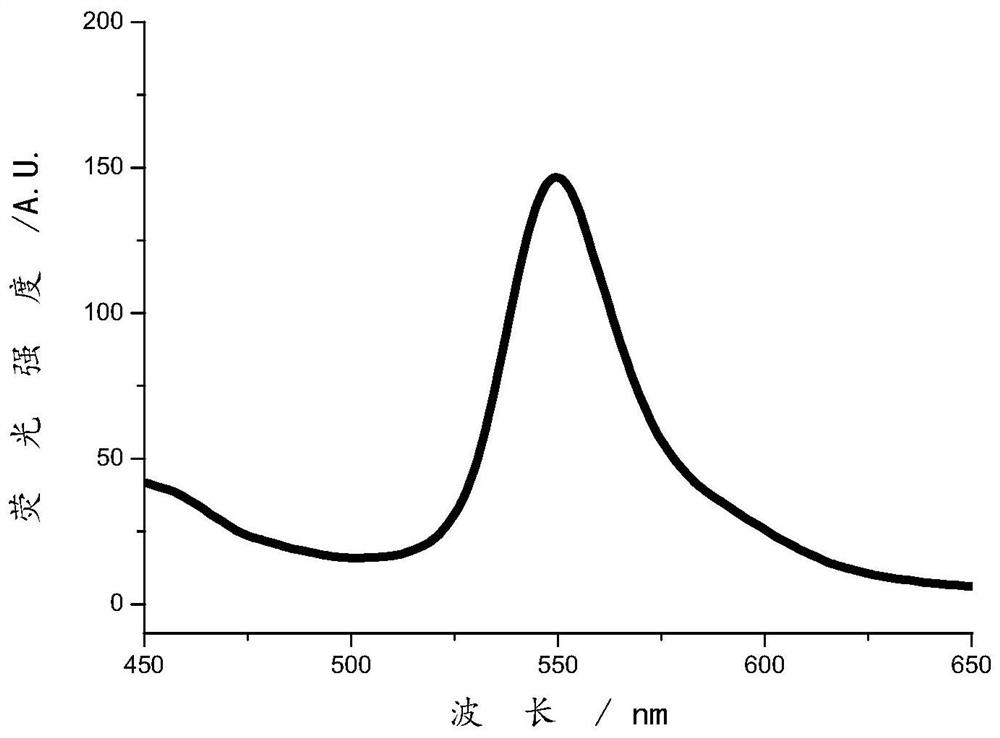
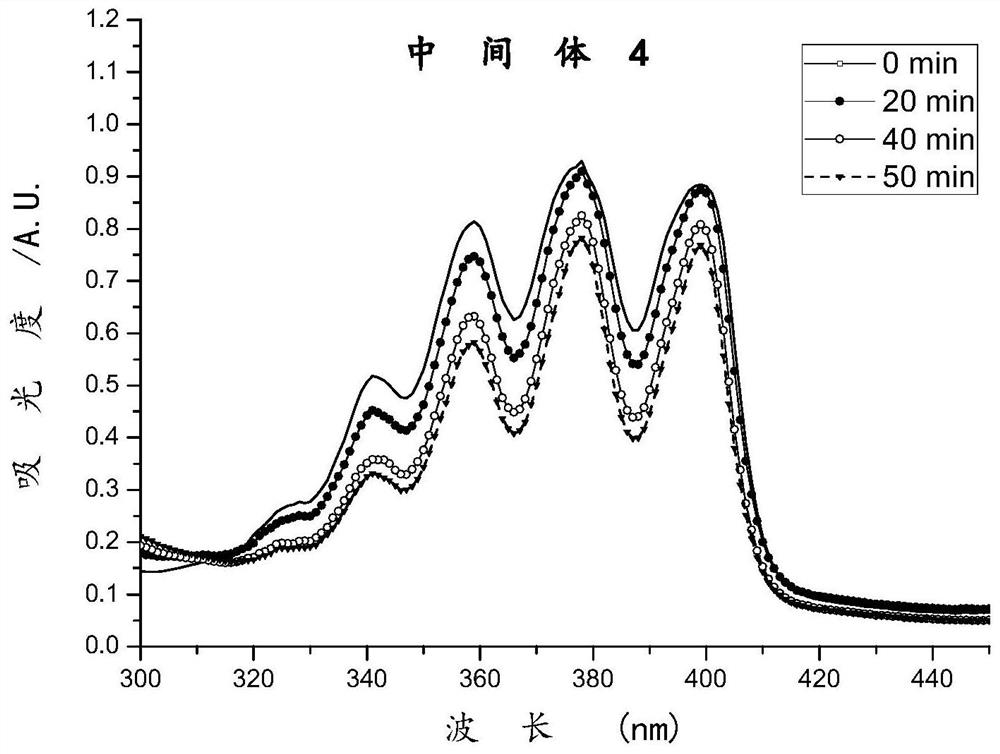
Image
Examples
Embodiment 1
[0031] (1) Under nitrogen atmosphere, dissolve 7-diethylamino-4-methylcoumarin (2.3g, 10mmol) in 12mL of N,N-dimethylformamide, then add N,N-di Methylformamide dimethyl acetal (2.6 mL); the reaction was refluxed overnight and cooled to room temperature;
[0032] Dichloromethane (50mL) and saturated sodium bicarbonate (50mL) were added to the reaction mixture, and the layers were shaken, the aqueous phase was extracted with dichloromethane (10mL×3), and the combined organic phases were dried over anhydrous sodium sulfate. Concentrated to remove organic solvents;
[0033] The residue was dissolved in a mixed solvent (60mL, THF:H 2 O=1:1), sodium periodate (6.2g, 29mmol) was added at room temperature to react for 2 hours, the filtered solid was washed with ethyl acetate until the washings were colorless, and the organic solvent in the filtrate was removed under reduced pressure , the residue was layered with dichloromethane (50mL) and saturated sodium bicarbonate (50mL), the aq...
Embodiment 2
[0049] (1) Under nitrogen atmosphere, dissolve 7-diethylamino-4-methylcoumarin (2.3g, 10mmol) in 12mL of N,N-dimethylformamide, then add N,N-di Methylformamide dimethyl acetal (2.6 mL); the reaction was refluxed overnight and cooled to room temperature;
[0050] Dichloromethane (50mL) and saturated sodium bicarbonate (50mL) were added to the reaction mixture, and the layers were shaken, the aqueous phase was extracted with dichloromethane (10mL×3), and the combined organic phases were dried over anhydrous sodium sulfate. Concentrated to remove organic solvents;
[0051] The residue was dissolved in a mixed solvent (60mL, THF:H 2 O=1:1), sodium periodate (6.2g, 29mmol) was added at room temperature to react for 2 hours, the filtered solid was washed with ethyl acetate until the washings were colorless, and the organic solvent in the filtrate was removed under reduced pressure , the residue was layered with dichloromethane (50mL) and saturated sodium bicarbonate (50mL), the aq...
Embodiment 3
[0067] Embodiment 3 of the present invention is basically the same as above-mentioned Embodiment 2, and difference points out to be:
[0068] (3) Under a nitrogen atmosphere, Intermediate 2 (738.2 mg, 2 mmol) and N-iodosuccinimide (9 mmol) were dissolved in ultra-dry dichloromethane (220 mL), and the mixture was stirred at room temperature for 24 h;
[0069] Pour into water (100mL) to separate the layers, the aqueous phase was extracted with dichloromethane (20mL×3), the combined organic phase was dried over anhydrous sodium sulfate, and concentrated to remove the organic solvent; the residue was purified by column chromatography to obtain a deep red Intermediate 3 (coordination complex 1: boron, difluoro[3-iodo-5-[(4-iodo-3,5-5-dimethyl-2H-pyrrole--2-alkylene-κN) (4-nitrophenyl))[methyl]-2,4-dimethyl-1H-pyrrolo-κN]-, (T-4)-coordination compound);
[0070] (4) Under a nitrogen atmosphere, add palladium carbon (0.6mmol), intermediate 3 (622.7mg, 1mmol), and hydrazine hydrate (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com