A kind of ACC inhibitor and its medicinal use
A pharmacy and drug technology, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problems of weak tumor cell activity and inability to meet clinical needs, and achieve excellent application prospects, significant in vitro proliferation inhibitory activity, and good anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
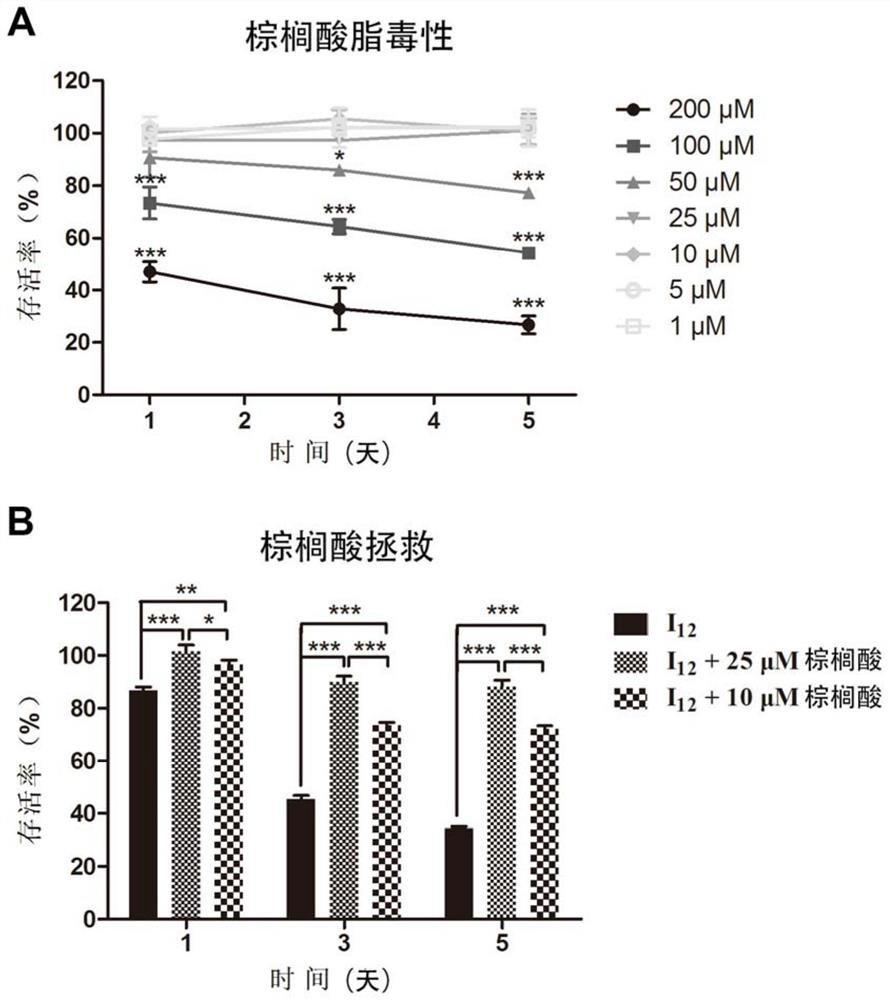
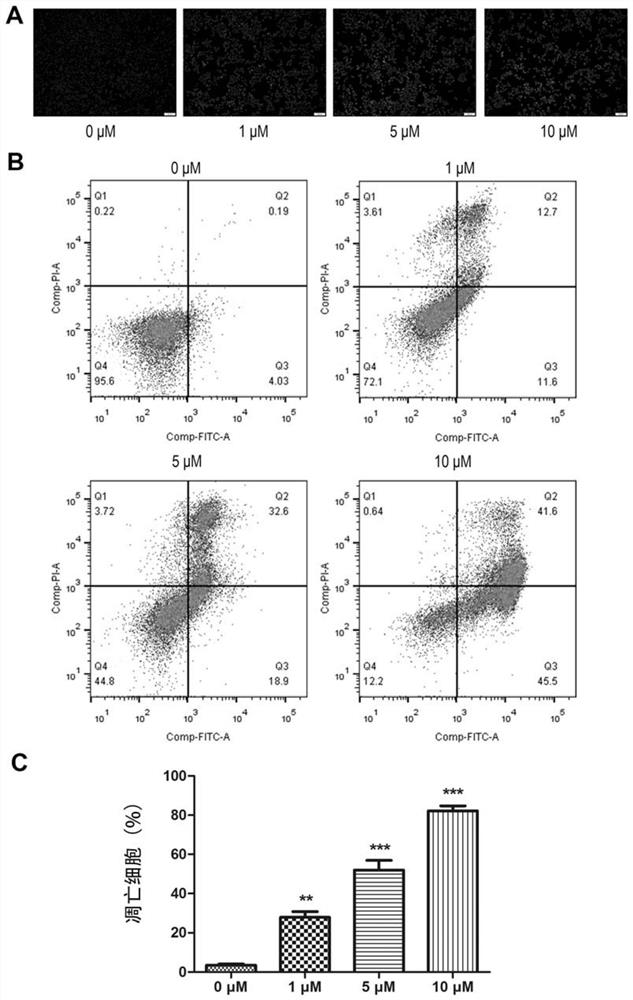
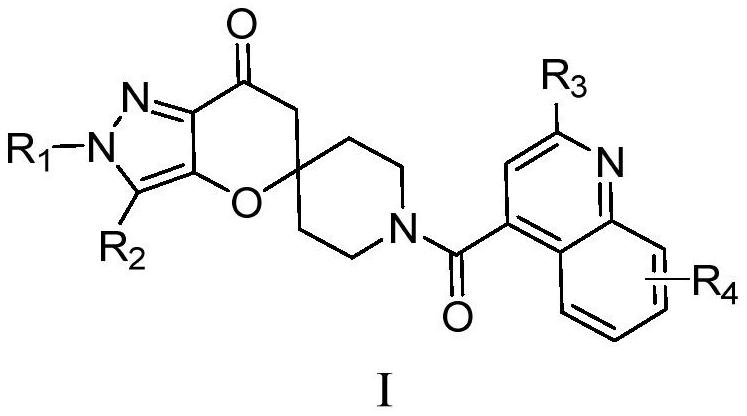
Image
Examples
Embodiment 1
[0102]
[0103] 2'-(tert-butyl)-1-(2-phenylquinoline-4-carbonyl)-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole ]-7'(6'H)-one (I 1 )
[0104] The aqueous solution (120 mL) of tert-butylhydrazine hydrochloride (3.74 g, 30 mmol) and glyoxal (4.5 g, 25 mmol) was reacted at room temperature for 2 h; the reaction solution was extracted with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and then reduced Concentrated under pressure to obtain compound (E)-1-(2-(tert-butyl)hydrazino)propan-2-one. (E)-1-(2-(tert-butyl)hydrazino)propan-2-one (8.8g, 62mmol) and 40% glyoxal solution (25mL, 180mmol) were dissolved in 100mL distilled water, heated to reflux for 5h After cooling to room temperature, the reaction solution was extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the compound 1-tert-butyl-4-hydroxypyrazole-3-ethanone. Dissolve 1-tert-...
Embodiment 2
[0107]
[0108] 2'-(tert-butyl)-1-(2-(p-methylphenyl)quinoline-4-carbonyl)-2'H-spiro[piperidine-4,5'-pyrano[3,2 -c]pyrazole]-7'(6'H)-one (I 2 )
[0109] According to the method of Example 1, the compound 2'-(tert-butyl)-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole]-7'( 6'H)-ketone. Under ice bath conditions, 2'-(tert-butyl)-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole]-7'(6'H )-ketone (400mg, 1.1mmol), HATU (458mg, 1.2mmol), triethylamine (0.2ml, 1.2mmol) were dissolved in 10mL DMF, stirred at 0°C for 0.5h, added 2-(p-methylphenyl) Quinoline-4-carboxylic acid (1 mmol, 265 mg) was reacted at room temperature for 12 h; the reaction solution was added to 50 mL of ice water, extracted with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the crude product was passed through a silica gel column Chromatographic purification to obtain the title compound I 2 .
[0110] Yellow solid, yield 80...
Embodiment 3
[0112]
[0113] 2'-(tert-butyl)-1-(2-(o-methylphenyl)quinoline-4-carbonyl)-2'H-spiro[piperidine-4,5'-pyrano[3,2 -c]pyrazole]-7'(6'H)-one (I 3 )
[0114] According to the method of Example 1, the compound 2'-(tert-butyl)-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole]-7'( 6'H)-ketone. Under ice bath conditions, 2'-(tert-butyl)-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole]-7'(6'H )-ketone (400mg, 1.1mmol), HATU (458mg, 1.2mmol), triethylamine (0.2ml, 1.2mmol) were dissolved in 10mL DMF, stirred at 0°C for 0.5h, added 2-(o-methylphenyl) Quinoline-4-carboxylic acid (1 mmol, 265 mg) was reacted at room temperature for 12 h; the reaction solution was added to 50 mL of ice water, extracted with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the crude product was passed through a silica gel column Chromatographic purification to obtain the title compound I 3 .
[0115] Yellow solid, yield 75...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com