A fluid method for the continuous preparation of iron oxide nanoparticles
A nano-fluid technology of iron oxide, applied in the direction of iron oxide, nanotechnology for materials and surface science, iron oxide/iron hydroxide, etc., can solve the problems of poor repeatability and uneven particle size, and achieve low cost and shape The effect of uniform appearance and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
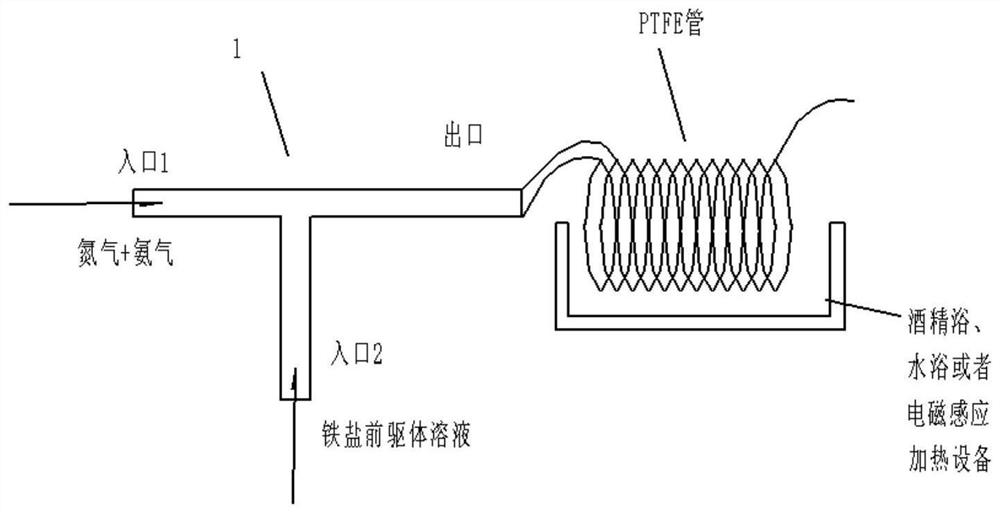
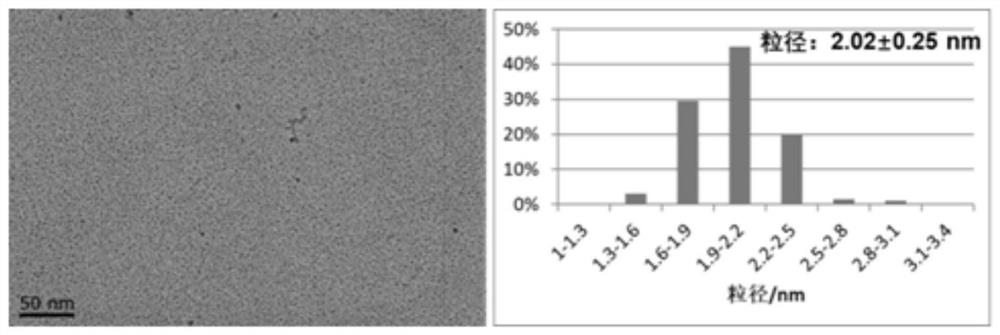
[0036]Ferrous chloride tetrahydrate (89.5 mg) and ferric chloride hexahydrate (243.3 mg) were ultrasonically dissolved in 20 ml ultrapure water, and the coating agent citric acid monohydrate (63 mg) was ultrasonically dissolved in 10 ml ultrapure water. The above solution was mixed ultrasonically and placed in a 50 ml syringe. Set the injection rate of the reaction solution to 500 μl / min, the flow rate of ammonia gas to 3.5 sccm, and the flow rate of nitrogen gas to 14 sccm. A PTFE tube with an inner diameter of 2.2 mm and a length of 10 m was connected to the reactor and placed in an alcohol bath at 1 °C. The residence time of the reaction solution in the PTFE tube was about 3 min. The schematic diagram of the reaction device and experimental implementation is as follows: figure 1 shown. Collect the product at room temperature and pass through 20 sccm nitrogen protection, use ultrapure water to wash the sample repeatedly by ultrafiltration until the solution is neutral, an...
Embodiment 2
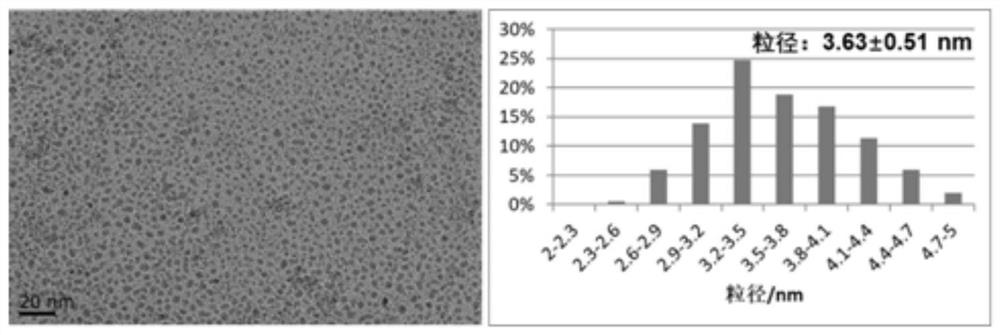
[0038] Ultrasonically dissolve ferrous chloride tetrahydrate (29.8 mg) and ferric chloride hexahydrate (81.1 mg) in 20 ml ultrapure water, and ultrasonically dissolve the coating agent PSC (90 mg) in 10 ml ultrapure water middle. The above solution was mixed ultrasonically and placed in a 50ml syringe. Set the injection rate of the reaction solution to 500 μl / min, the flow rate of ammonia gas to 3.5 sccm, and the flow rate of nitrogen gas to 14 sccm. A PTFE tube with an inner diameter of 2.2 mm and a length of 10 m was connected to the reactor and placed in an alcohol bath at 1 °C. The residence time of the reaction solution in the PTFE tube was about 3 min. Collect the product at room temperature and pass through 20 sccm nitrogen protection, use ultrapure water to wash the sample repeatedly by ultrafiltration until the solution is neutral, and obtain PSC-modified iron oxide nanoparticles with uniform particle size and morphology distribution, such as image 3 shown. The ce...
Embodiment 3
[0040] Ultrasonically dissolve ferrous chloride tetrahydrate (29.8 mg) and ferric chloride hexahydrate (81.1 mg) in 30 ml ultrapure water, and place the reaction solution in a 50 ml syringe. Set the solution injection rate to 2 ml / min, the ammonia gas flow to 5 sccm, and the nitrogen gas flow to 15 sccm. A PTFE tube with an inner diameter of 2.2 mm and a length of 15 cm was connected to the reactor and placed at room temperature. The residence time of the reaction solution in the PTFE tube was about 3 s. The product was directly passed into a three-necked flask, the product was collected at room temperature and protected by 20 sccm nitrogen, and the reaction was continued to stir at 600 rpm for 1 h. After the reaction is over, use ultrapure water to wash the sample repeatedly through magnetic separation until the solution is neutral to obtain unmodified bare iron oxide nanoparticles, such as Figure 4 shown. The magnets used for magnetic separation are NdFeB magnets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Resonant frequency | aaaaa | aaaaa |
| power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com