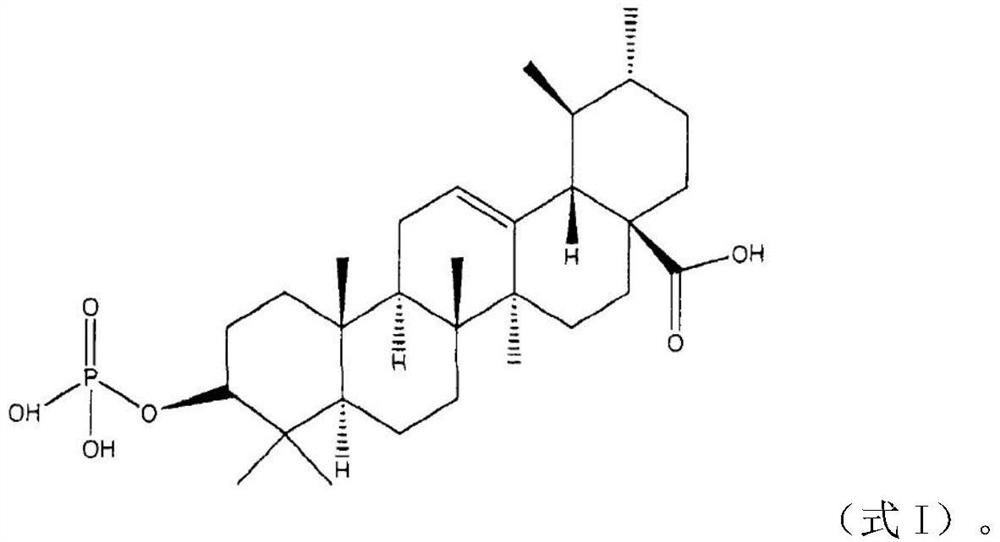
Application of jzy-13 and compound in preparation of medicament for treating psoriasis
A compound and technology for psoriasis, which is applied in the field of pharmaceuticals for the treatment of psoriasis, can solve the problems of insufficient and insufficient keratinocytes, and achieve good market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Isolation and culture of keratin forming cells
[0022] Take the leather tissue after menopausculectomy, immediately put it in PBS with gentamicin, amphotericin, and then remove blood after surgery. In advance, the horny formation of HKGS, chimmycin / amphotericin (500x) is added, and the tissue is placed in the culture medium, remove skin tissue, cutting the skin tissue into small pieces, about 5mm x5mm, plus DISPASE II Enzyme (1.2U / mL) is 4 ° C to prevent light overnight; the epidermis and leather were separated, and the epidermal was digested with 0.5% pancreatic enzyme at 37 ° C, and the time was about 5-lomin, and the DMEM medium containing 10% serum was digested. The 200 mesh is ground, filtered, centrifuged, and PBS washed twice, using antibiotic epidermal cell culture medium retransmitted, paving; 3-4 days of cells can be attached, fibroblasts are not growing in epidermal medium, The epithelial cells are gadily in the formation, and the cell fusion reach...
Embodiment 2
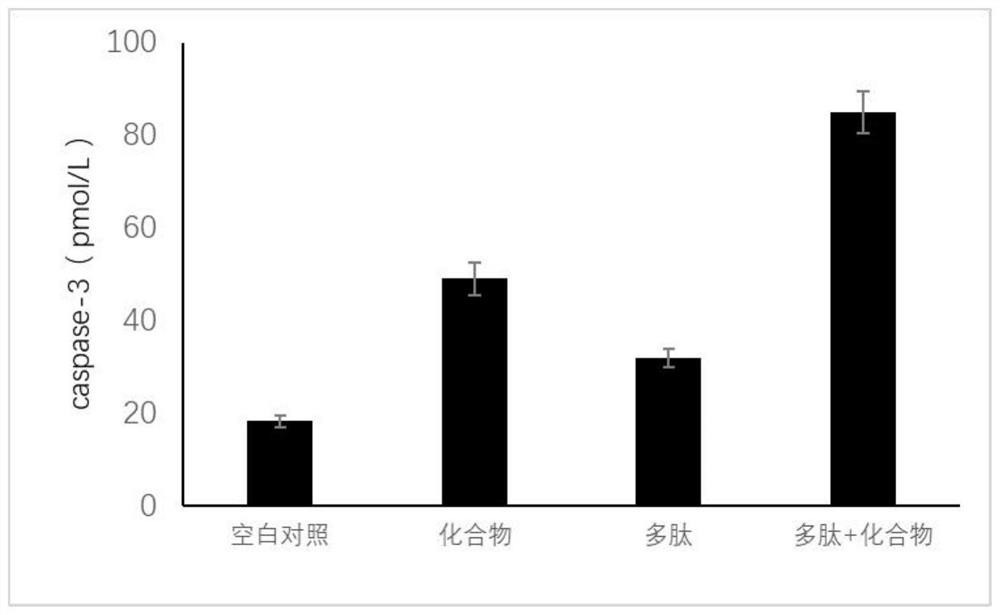
[0023] Example 2 Preparation of keratin forming cell inhibitor compounds
[0024] Usopine (48.1 g, 0.211 mol), dimethyl-N, N-diethyl amino phosphate (34.82 g, 0.211 mol), a mixture of dried tetrahydrofuran (1250 mL) was heated to 35 ° C to prepare a transparent solution, and 1H-tetrazole (44.25 g, 0.632 mol) was added at a time at 27 ° C, and then stirred at room temperature (22 ° C) for 1 hour. After the addition of phosphite dimethyl esters were confirmed by TLC, a acetone-dry ice was cooled with acetone-dry ice, and an aqueous solution (84 ml, 0.607 mol) of 70% tert-butyl hydroperoxide was added dropwise therein. The ice bath was removed, gradually warmed to room temperature, and TLC was subjected to TLC to confirm the disappearance of the phosphite and the production of phosphoric acid dimethyl, and then the reaction was terminated with aqueous solution of 10% sodium sulfite at 0 ° C. Ethyl acetate (1250 mL) was added to the reaction solution and the organic layer was separate...
Embodiment 3
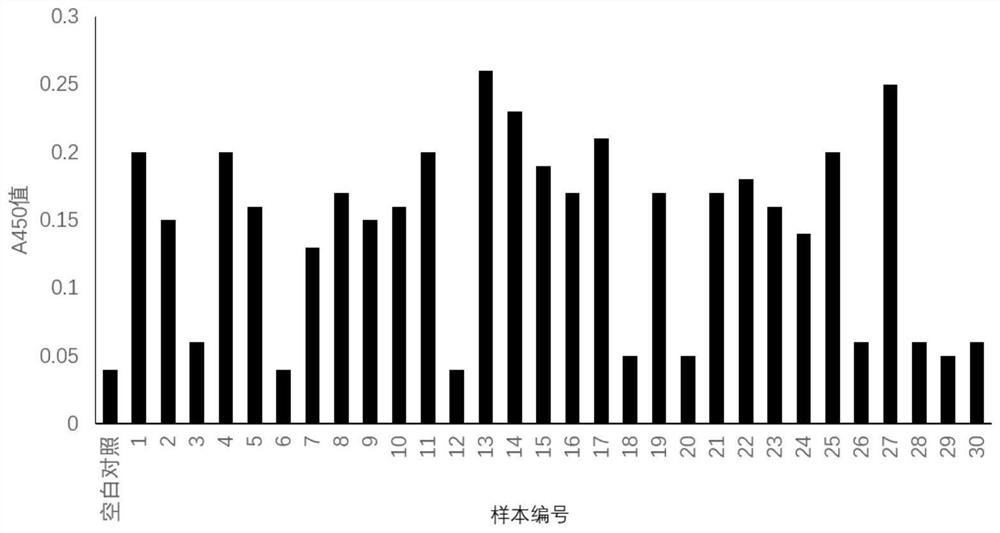
[0025] Example 3 Oral forming cell inhibitor polypeptide screening
[0026]Phage random 12 peptide kit kit (Ph.D.TM-12PHAGE DISPLAY PEPTIDE LIBRARY KIT) was purchased from NEW ENGLAND BIOLABS, and the host bacteria were E.COLI ER2738.
[0027] Random doubral peptide phage display library biometric screening: Pretreatment of microplate using 0.1 mol / L Multi-lysine, 1) Package: Oral forming cells prepared by Example 1 1 × 10 6 The / ml package was overnight by a microplate, a wet cartridge in a 4 ° C, and then added to the fixed liquid (glutaraldehyde), then washed 3 times; 2) binding: blocking buffer [0.1 mo l / lnahco 3 (pH8.6), 5mg / ml BSA, 0.02% NaN 3 [Closed, TBST washing board 6 times, 10 μL of the original library was diluted with 90 μl of TBST, and the microporous was added, and the temperature was mild and shaken for 60 min. 3) Elution: Turn off the liquid, wash the plate 10 times, add 0.2 mol / l glycine-hydrochloric acid buffer (pH2.2) 100 μl / hole, and shake the room...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com