Electrolytic gold plating solution, method of manufacturing the same, gold plating method and gold complex
A technology of gold complexes and manufacturing methods, applied in the direction of gold organic compounds, 1/11 group organic compounds without C-metal bonds, etc., can solve the problems of difficult management, reduced oxidation stability of gold complexes, and low oxidation stability and other problems, to achieve the effect of good current efficiency and excellent oxidation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0126] Hereinafter, the present invention will be described more specifically with reference to examples, but the present invention is not limited to these examples.
[0127]
[0128] [Extraction of Hydantoin Gold Complex Sodium Salt]
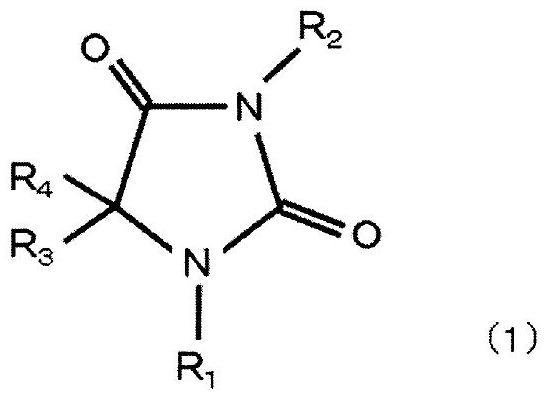
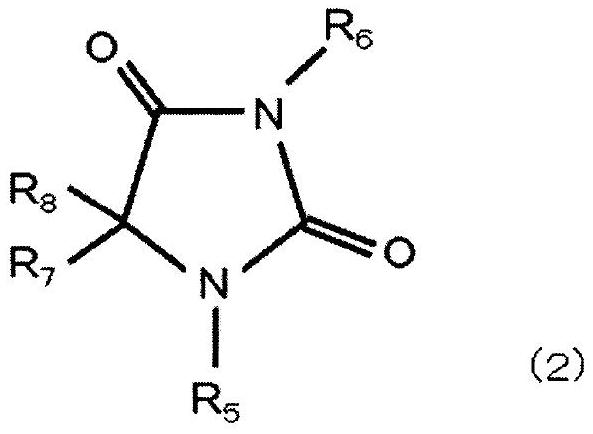
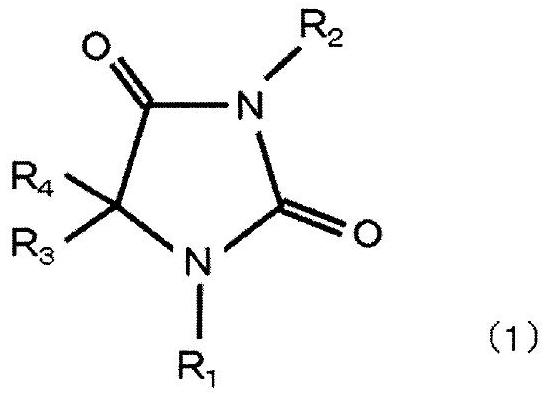
[0129] Dissolve 50 g of hydantoin in 250 mL of water to form an aqueous hydantoin solution, adjust the aqueous hydantoin solution to pH 11.5 to 12.5 with sodium hydroxide, and then add 25 g of chloroauric acid in terms of gold (HAuCl 4 ), heated and stirred at 65° C. for 180 minutes to react, and a hydantoin gold complex was obtained.
[0130] Next, sodium hydroxide was further added to the aqueous solution containing the obtained gold complex so that the pH was 9.0, and the aqueous solution was cooled to room temperature (25° C.) or lower to precipitate crystals of hydantoin gold complex sodium salt. Then, hydantoin gold complex sodium salt, which is an alkali metal salt of a gold complex, was extracted from the aqueous solution by filtrat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap