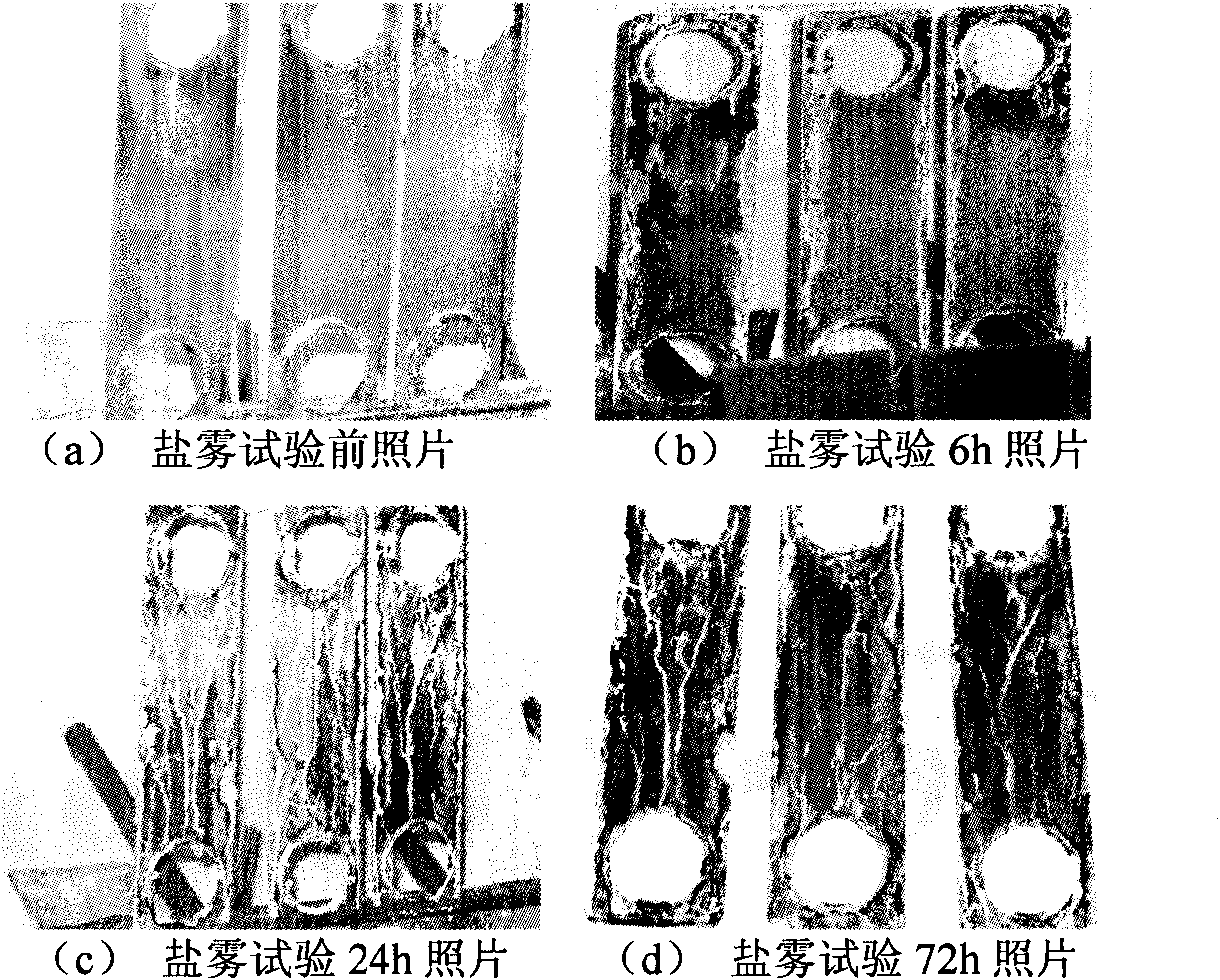
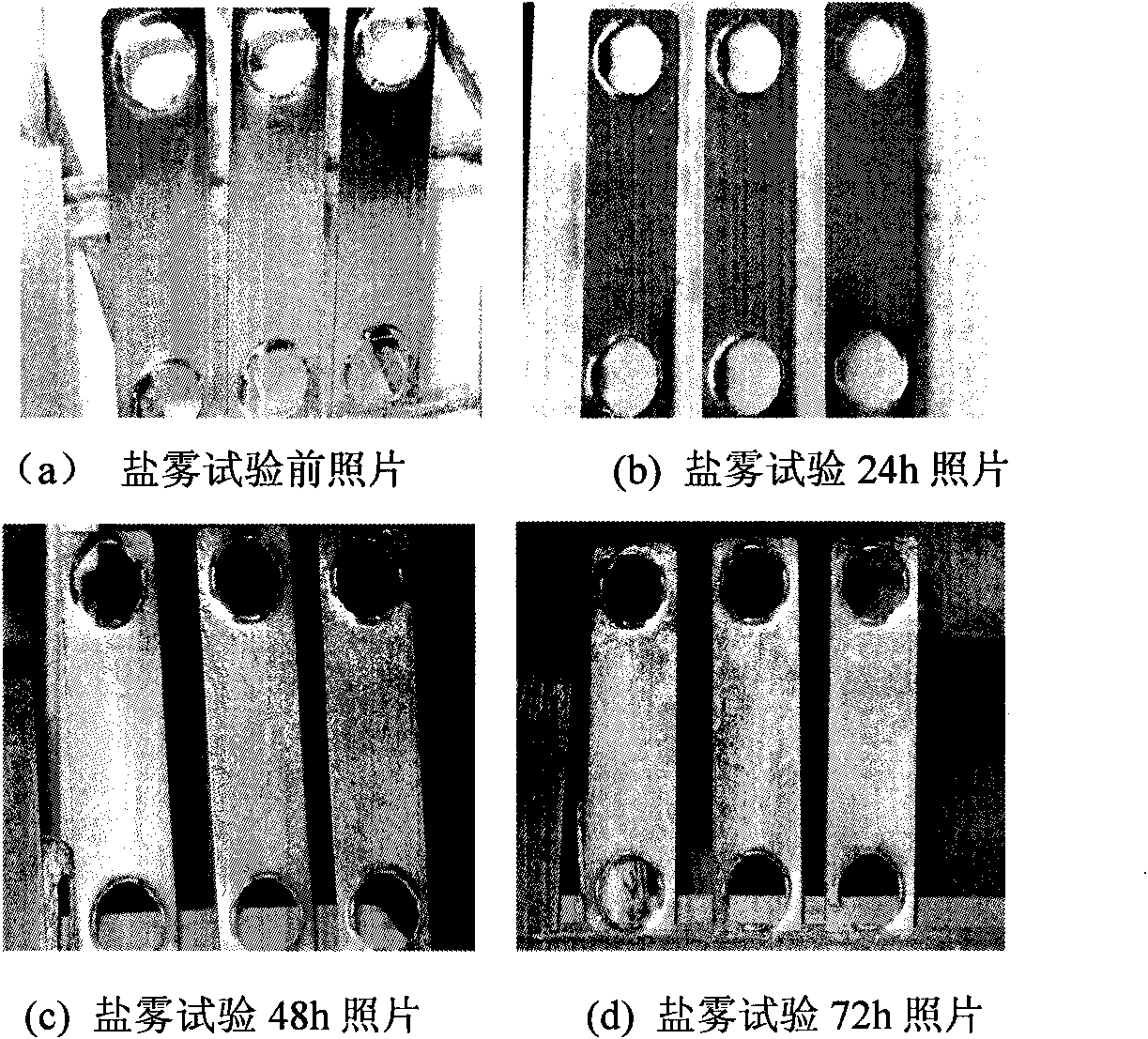
Non-cyanide converting method for cyanide plated zinc
A technology of cyanide and zinc oxide, applied in the field of electrolysis or electrophoresis on metal surface to produce coating, can solve problems such as affecting the quality of electroplating, scrapping of electroplating bath, difficult to remove, etc., achieving obvious environmental benefits, strong resistance to impurities, environmental protection small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
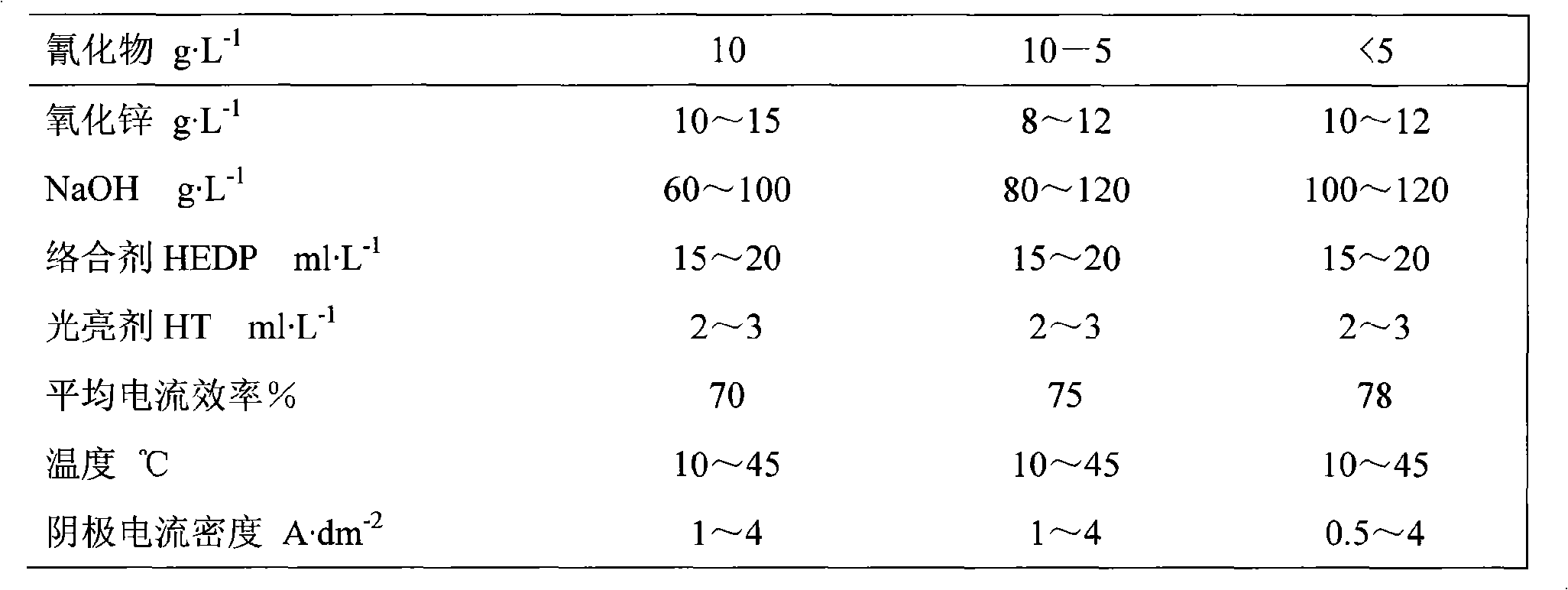
[0051] Cyanide plating conditions:
[0052] Cyanide content g·L -1 10
[0053] Zinc oxide content g·L -1 10~15
[0054] NaOH contentg·L -1 60~100
[0055] Complexing agent HEDP ml·L -1 15~20
[0056] Brightener HT ml·L -1 2~3
[0057] Temperature ℃ 15~45
[0058] Cathode current density A·dm -2 1~4
[0059] The average current efficiency of electroplating is 70%.
Embodiment 2
[0061] Cyanide plating conditions:
[0062] Cyanide content g·L -1 10~5
[0063] Zinc oxide content g·L -1 8~12
[0064] NaOH contentg·L -1 80~120
[0065] Complexing agent HEDP ml·L -1 15~20
[0066] Brightener HT ml·L -1 2~3
[0067] Temperature ℃ 15~45
[0068] Cathode current density A·dm -2 1~4
[0069] The average current efficiency of electroplating is 75%.
Embodiment 3
[0071] Cyanide plating conditions:
[0072] Cyanide content g·L -1 <5
[0073] Zinc oxide content g·L -1 10~12
[0074] NaOH contentg·L -1 100~120
[0075] Complexing agent HEDP ml·L -1 15~20
[0076] Brightener HT ml·L -1 2~3
[0077] Temperature ℃ 15~45
[0078] Cathode current density A·dm -2 0.5~4
[0079] The average current efficiency of electroplating is 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com