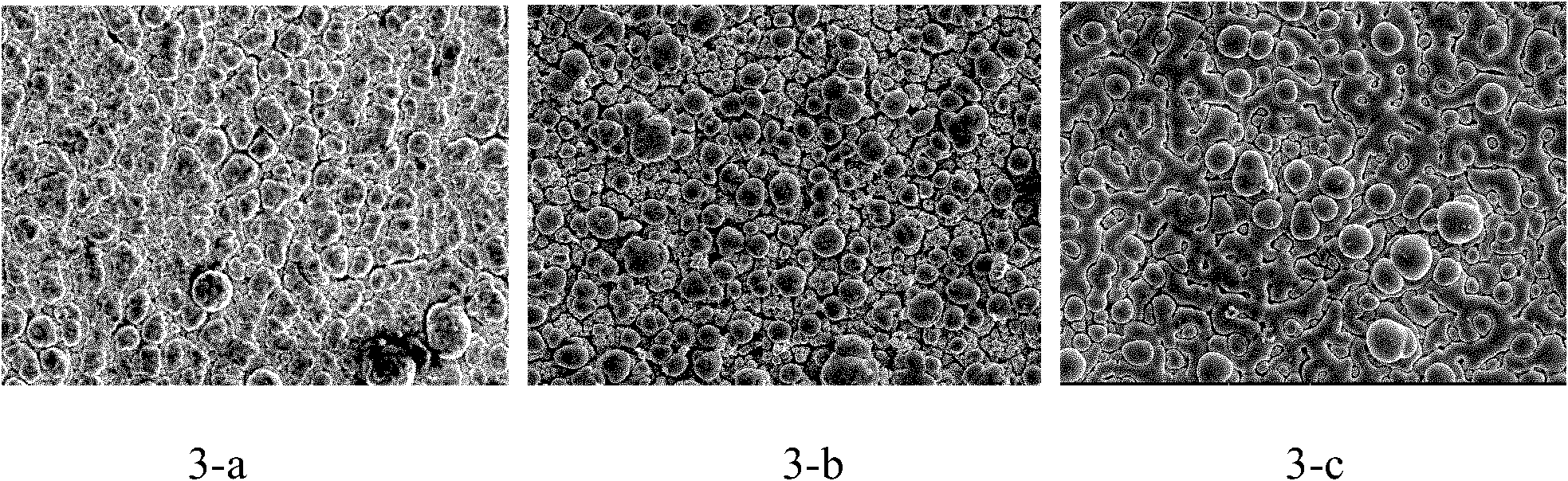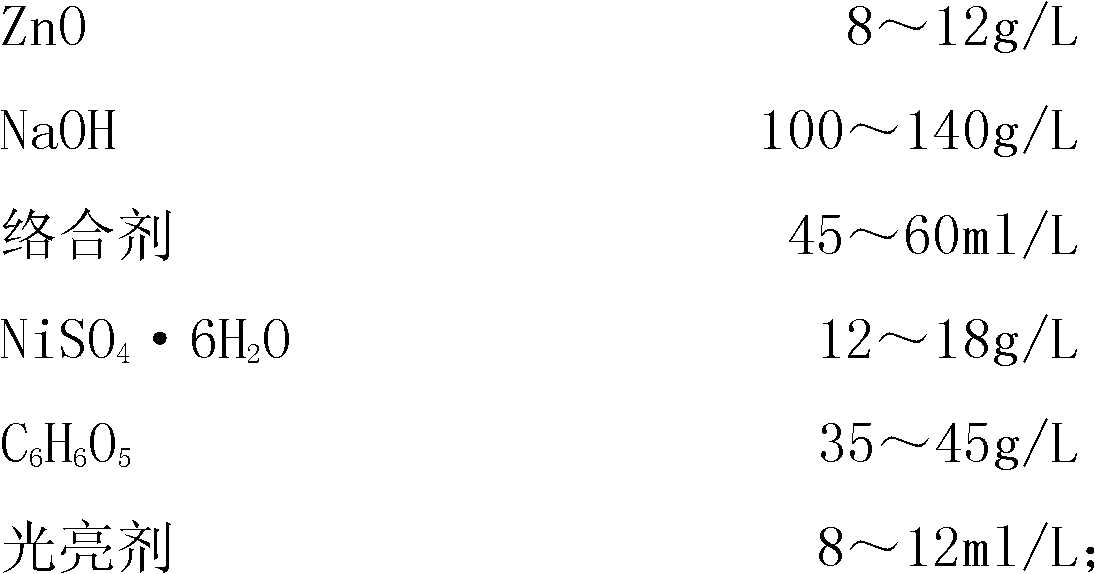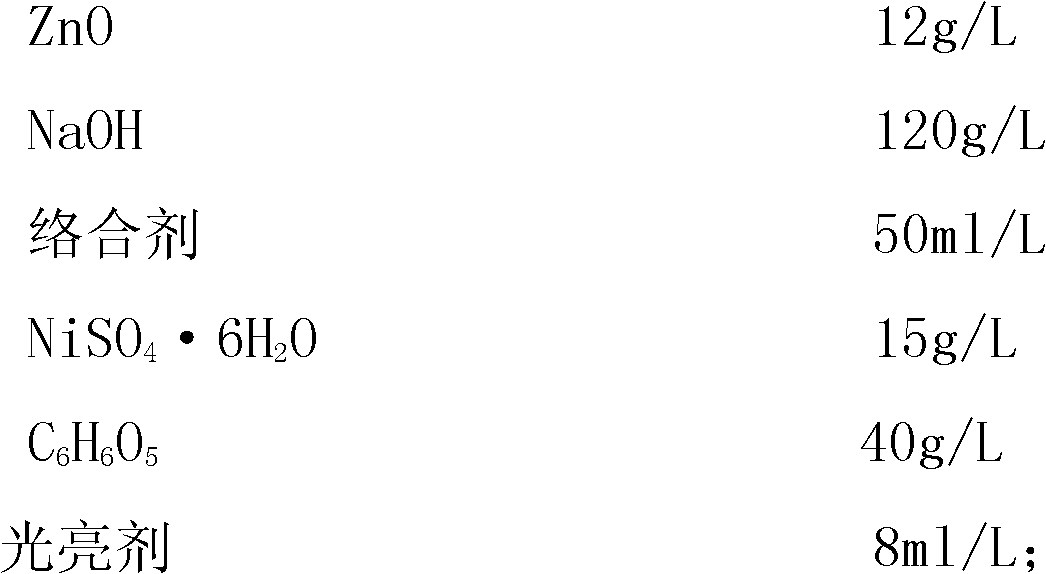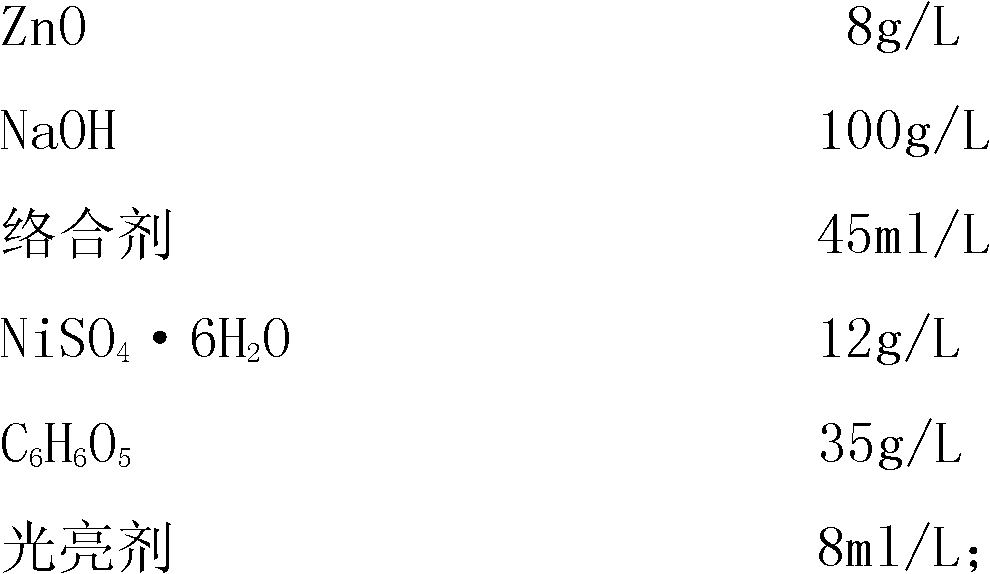Patents
Literature
245 results about "Cathode current density" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A. You can set the required current density by knowing first the area of the cathode (or anode), then multiply it to your desired current density. The product would be your current setting. In a chromium electroplating process it says the cathode current density is 20-30 A/dm2.
Rare earth aluminum alloy, and method and device for preparing same
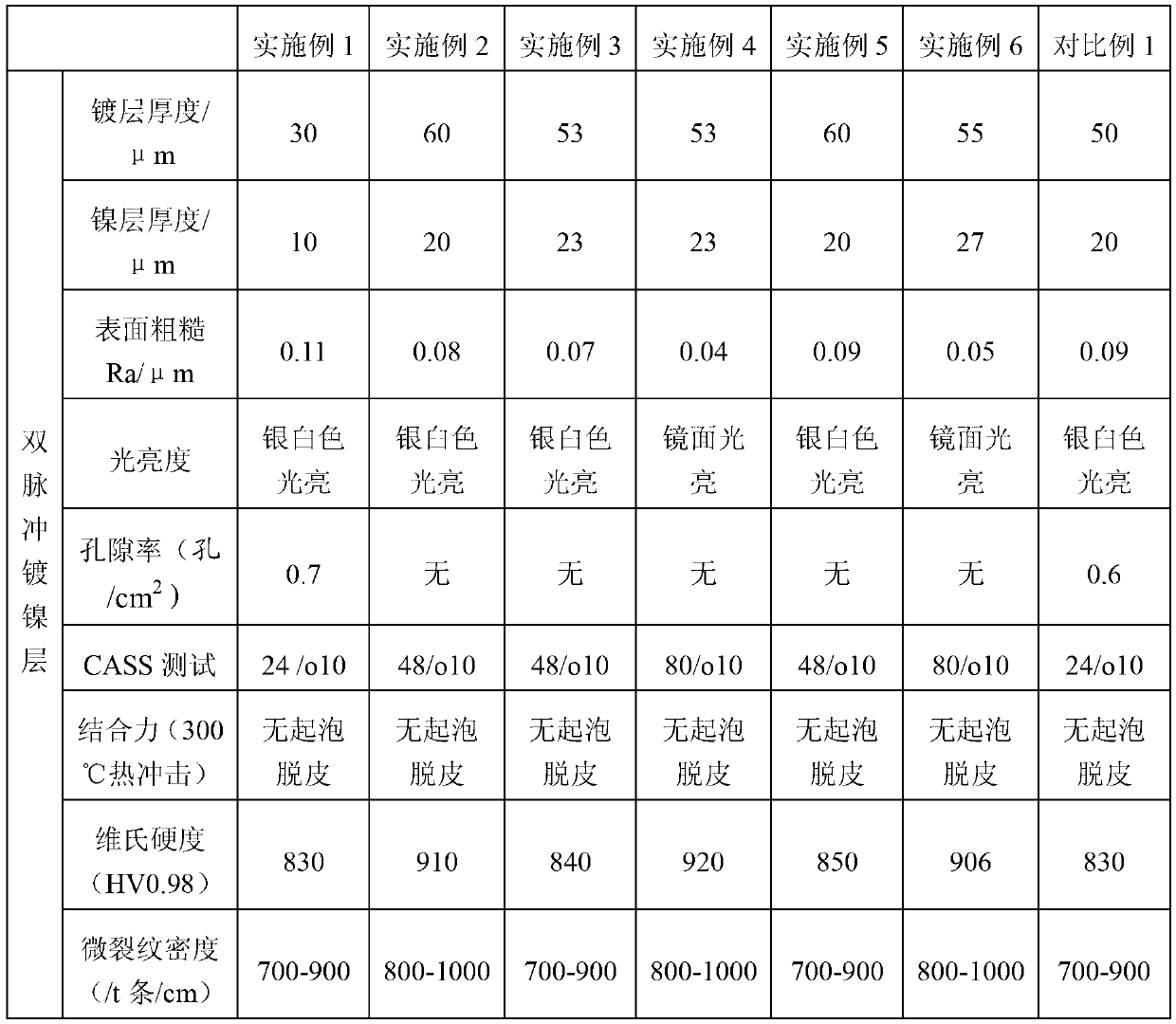
The invention discloses a rare earth aluminum alloy, and a method and a device for preparing the same. The alloy contains at least one rare earth metal of lanthanum, cerium, praseodymium, neodymium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, lutetium, scandium and yttrium, the content of raw earth is 5 to 98 weight percent, and the balance is aluminum and inevitable impurities. The device for preparing the rare earth aluminum alloy is characterized in that: a) graphite serves as an electrolysis bath, a graphite plate is an anode, a tungsten bar is a cathode and a molybdenum crucible serves as a rare earth aluminum alloy receiver; b) the diameter of the tungsten bar is 30 to 55 mm; and c) the anode of the graphite consists of a plurality of graphite plates. The rare earth aluminum alloy, and the method and the device for preparing the same have the advantages that: the alloy has uniform components, little segregation and low impurity content; technology for preparing the rare earth aluminum alloy through fusion electrolysis can maximally replace a process for preparing single medium-heavy metal through metallothermic reduction, greatly reduce energy consumption and the emission of fluorine-containing tail gas and solid waste residue, improve current efficiency and metal yield and reduce the consumption of auxiliary materials and the energy consumption; and the rare earth aluminum alloys with different rare earth contents can be obtained by controlling different electrolytic temperatures and different cathode current densities.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Multi-functional half-white brightness tin-plated additive
The invention provides a multi-functional half-white brightness tin-plated additive which comprises the following components in parts by weight: 0.1-3 parts of a main brightener, 5-15 parts of a dispersing agent, 0.1-3 parts of a stabilizing agent, 0.1-2 parts of a leveling agent, 0.1-3 parts of a change agent, 0.1-2 parts of a low-foam wetting agent, 1-5 parts of an anti-crystal whisker agent, 0.05-0.5 part of a flocculating agent, 40-55 parts of an aqueous organic solvent, 0.1-2 parts of a functional auxiliary, 0.1-2 parts of acid and 25-40 parts of water. The multi-functional half-white brightness tin-plated additive has good dispersive capacity and coverage capacity, can enable the plating layer thickness to reach 3 micrometers or more, is fine, smooth and compact in crystal grains and has high bonding strength with a matrix; according to the multi-functional half-white brightness tin-plated additive, the performance of a plating layer can be obviously improved, the cost of a plating solution is low, and the electroplating liquid has wide operating temperature range and cathode current density range; the tin plating layer is compact is surface structure, high in white brightness and strong in bonding strength and has excellent weldability and oxidation resistance.
Owner:苏州禾川化学技术服务有限公司
High-current density metal electrolytic deposition device with bottom inlet liquid circulation and realization method thereof
ActiveCN104032332AIncreased unit production capacityEvenly distributedPhotography auxillary processesElectrolysis componentsHigh current densityConcentration polarization
The invention relates to a high-current density metal electrolytic deposition device with bottom inlet liquid circulation and a realization method thereof. The device comprises an electrolytic tank, cathode plates and anode plates, wherein conducting rods are arranged on the cathode plates and the anode plates; the cathode plates and the anode plates are accommodated in the electrolytic tank; a circulating flow liquid inlet device is mounted at the bottom of the electrolytic tank, and comprises a circulating tank, a solution circulating channel, a circulating pump, a lower tank and an overhead tank; the lower tank and the overhead tank are connected through an extraction-reverse extraction system; the lower tank is connected with one end of the circulating tank through an overflow pipe; and the overhead tank is connected with the other end of the circulating tank through a pipe to form circulation. The device has the following benefits: the device accelerates the migration speed of metal cation through increasing the circulating flow of catholyte to eliminate the cathode concentration polarization and to realize higher cathode current efficiency and high-quality cathode products under the condition of high cathode current density, so that the purpose of prominently improving the production capacity of unit electrolysis devices is achieved through largely increasing the current density.
Owner:HANGZHOU SANAL ENVIRONMENTAL TECH
Preparation method of high-hardness Cu-SiC nanometer compound plating layer and special device thereof
InactiveCN101717977AHigh content of nanoparticlesEvenly distributedElectrolytic coatingsNanoparticleCrystal structure
The invention discloses a preparation method of a high-hardness Cu-SiC nanometer compound plating layer and a special device thereof. The preparation method comprises the following steps of: pretreating nanometer SiC particles, preparing an electrolytic solution containing the nanometer SiC particles, primarily treating a cathode plate and carrying out electrolytic deposition for preparing the high hardness Cu-SiC nanometer compound plating layer. In the preparation method of a high-hardness Cu-SiC nanometer compound plating layer, secondary reunion of the nanometer particles can be effectively avoided, the electrolytic solution spray deposition process can be higher than the cathode current density of the common electrodeposition processes for carrying out electrolytic deposition, and therefore, high speed electrolytic deposition on unit area of the cathode surface can be realized. The nanometer composite plate prepared by the invention has the advantages of high content of nanometer particles, uniform distribution of nanometer particles, high hardness, compact crystal structure, good surface quality, and the like.
Owner:HUAIHAI INST OF TECH
Method for preparing neodymium-iron-boron magnetic powder coated with metal layer by electrochemical deposition
InactiveCN1690255AIncreased corrosion potentialImprove magnetic propertiesMagnetic materialsMetal sheetElectrochemistry
The invention provides a method for electrochemical deposition cladding metal sheet of Nd-Fe-B magnetic particle, that is: electrodeposition solution containing metallic ion in electrolyser, laying the degreased and activated Nd-Fe-B magnetic particle on the conductive negative plate, the metallic plate corresponding to the related electrodeposition solution as the anode, galvanizing in a cathode current density of 0.1 to 5 A / dm2 and a temperature of 10 to 50 Deg. C to make the surface of Nd-Fe-B magnetic particle coat sheet metal, separating and purifying, then the Nd-Fe-B magnetic particle the surface of which is coated with sheet metal prepared. The invention has a characteristic of modifying the surface of magnetic particle in a low temperature, increasing largely the corrosion electric potential, and advanced the magnetic property, oxidation resistance property and conductivity. With electrochemical deposition technology, the technique of coating sheet metal is simple, and the thickness of metal cladding can be controlled randomly.
Owner:ZHEJIANG UNIV OF TECH
Non-cyanide alkaline copper plating bath, preparation and use method thereof
The present invention is a new non-cyanide alkaline copper plating bath, preparation and use method thereof. In the copper plating bath, using copper sulfate or basic copper carbonate as main salt, using hydroxy-ethylidene diphosphonic acid as main complexant, using trisodium citrate, potassium citrate or potassium sodium tartrate as auxiliary complexant, using sodium nitrate or potassium nitrate as conductive salt, using sodium hydroxide or potassium hydrate as pH value regulator; the operation conditions are: cathode current density is 0.5-3.0 A / dm [2], pH of plating bath controlled between 12 and 13, plating bath temperature is 50-70 DEG. Comparing with the prior known technology, the invention has following advantages or positive effects: simple plating bath formula, easy control and operation, wide temperature range of plating bath using, high current efficiency, fine crystallization coating, good appearance color, stable plating bath, strong uniform plating and covering ability, low cost, easy wastewater treatment. The invention can be used for pre copper plating or direct electro-coppering instead of virulent cyaniding electro-coppering process.
Owner:KUNMING UNIV OF SCI & TECH
Wear-resistant worpiece and manufacturing method of wear-resistant coating thereof
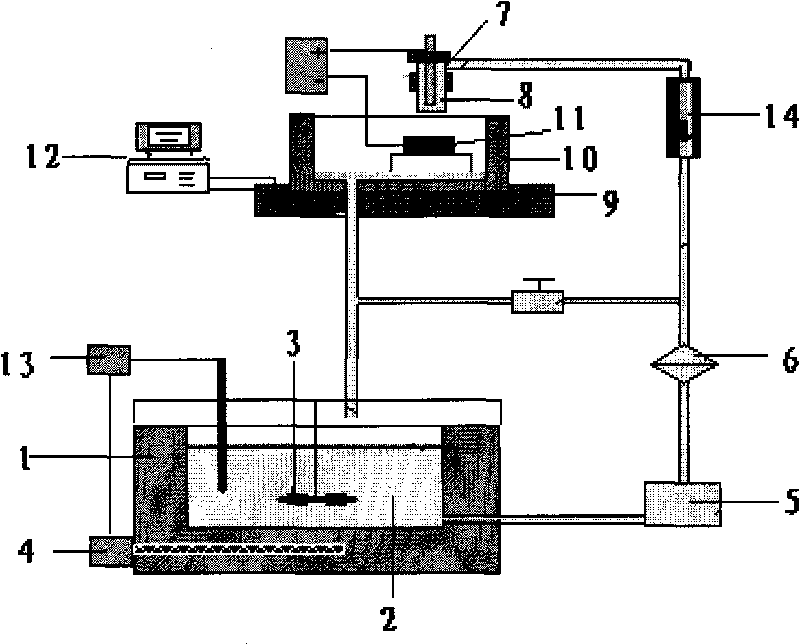
The invention discloses a wear-resistant workpiece and a manufacturing method of a wear-resistant coating thereof. The preparation method of the wear-resistant coating comprises the following steps: forming a preparation piece according to the structure of a required wear-resistant workpiece; coating nickel on the surface of the preparation piece through a double-pulse method, thus forming a nickel-coated transition piece; and coating a hard chromium layer on the surface of the nickel-coated transition piece, thus forming the wear-resistant coating on the surface of the preparation piece. For the manufacturing method of the wear-resistant workpiece, a step of coating nickel on the surface of the workpiece through a double-pulse nickel coating method is added; and the double-pulse nickel coating method is characterized in that the magnitude of current or voltage is regulated by an external control means, the current is additionally controlled by controlling the pulse switch-on time, the pulse switch-off time, the pulse duty factor, the pulse current density and the like, and corresponding variables are changed to respectively achieve the effects of increasing the cathode current density, inhibiting the generation of side reaction, reducing the impurity content in the coating, improving the current distribution and the like, thus improving the quality of the coated nickel layer and prolonging the service life of the wear-resistant workpiece.
Owner:HUNAN TELI HYDRAULIC +1
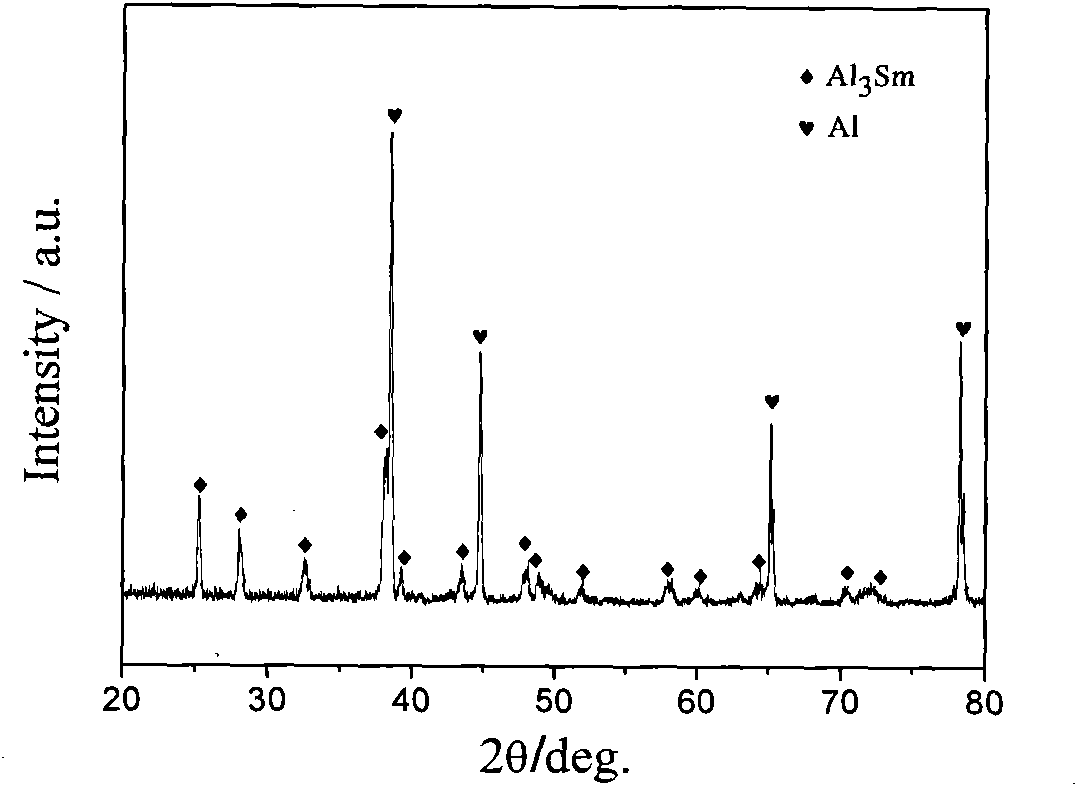
Aluminum-lithium-samarium alloy and fused salt electrolysis preparation method thereof
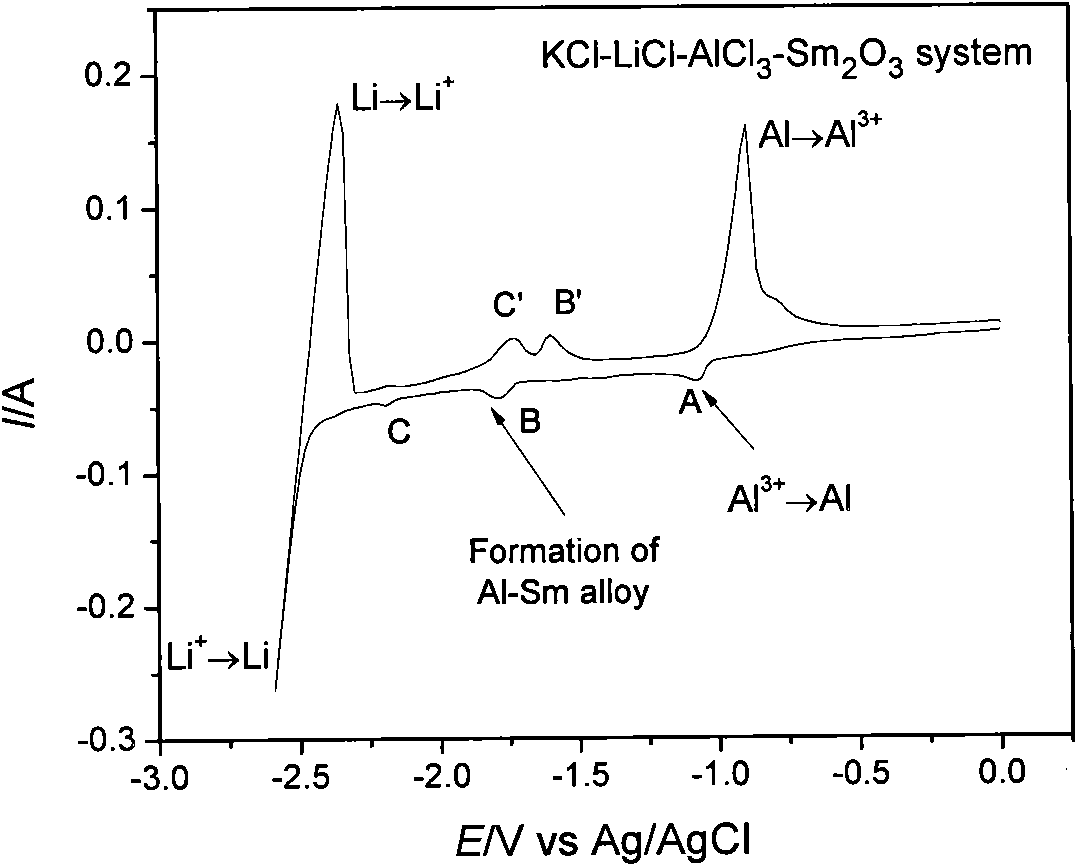
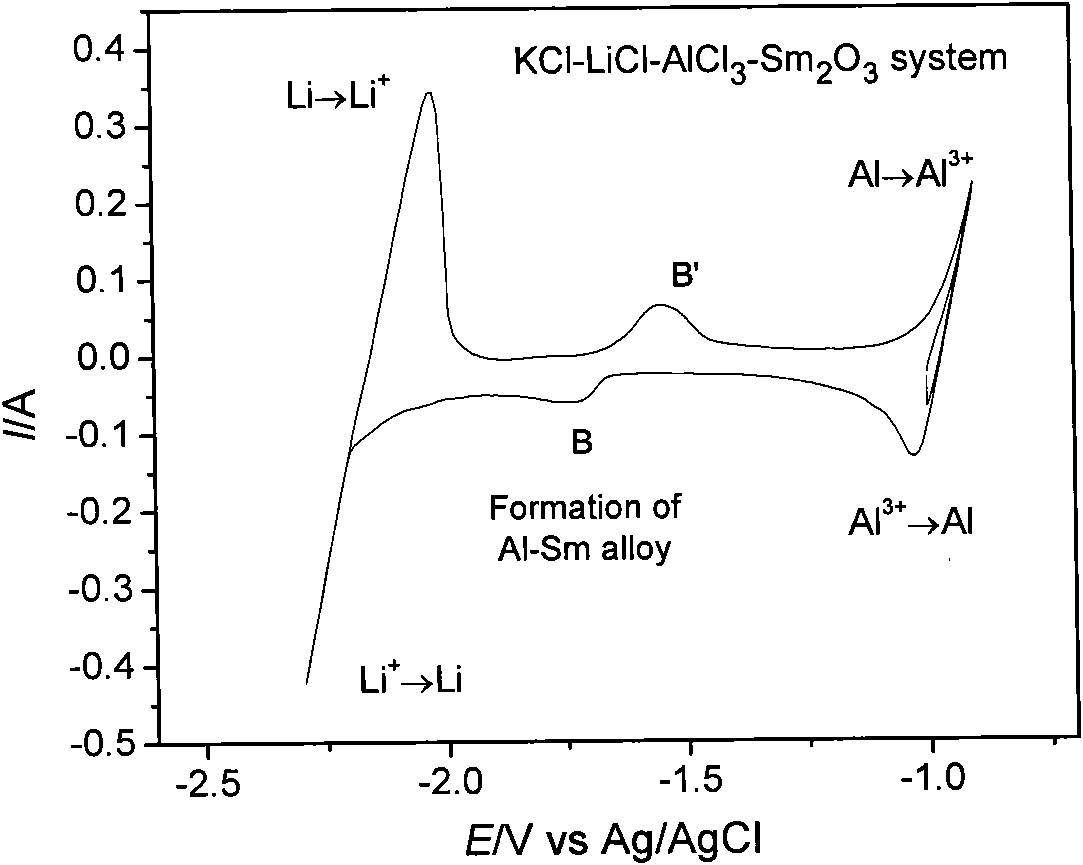
The invention provides an aluminum-lithium-samarium alloy and a fused salt electrolysis preparation method thereof. The aluminum-lithium-samarium alloy is prepared by the following steps of: heating LiCl and KCl serving as an electrolyte system to 630 DEG C for fusion in an electrolytic furnace; uniformly mixing Sm2O3 powder and AlCl3 and tabletting the mixture, adding the mixture into fused salt in the form of grains to ensure that the mass ratio of the AlCl3, LiCl to KCl is 6.2-11.0 percent to 44.5-46.9 percent to 44.5-46.9 percent, wherein the amount of the added Sm2O3 is 1 percent of the weight of the electrolytic fused salt; and taking metal molybdenum as a cathode and graphite as an anode and performing electrolysis for 2 to 6 hours to deposit the Al-Li-Sm alloy close to the cathode of the fused salt electrolysis cell, wherein the electrolysis temperature is between 630 and 720 DEG C, the cathode current density is 6.4A / cm<2> and the anode current density is 0.5A / cm<2>. By completely using metal compounds as raw materials and adding aluminum chloride, the chlorination of samarium oxide is realized and the aluminum-lithium-samarium alloys of different components are obtained by controlling the conditions such as the proportion of the electrolytes, the electrolysis time, the temperature, the current density and the like. The alloy and the method have the advantages of simple whole process, low requirements on equipment, low energy consumption and low pollution.
Owner:HARBIN ENG UNIV
Manufacturing method of metal-coated polyimide substrate
ActiveCN101350315AReduce dispersionImprove failure rateSemiconductor/solid-state device manufacturingConductive pattern formationPolyimide substrateSurface resistivity
The invention provides a metal faced polyimide base plate manufacturing method capable of reducing the dimensional change dispersibility while heating the metal faced polyimide base plate, jointing stably under the endured heat quantity when used as COF and improving the qualification rate. The method comprises: a sputtering process for forming a metal coat on the polyimide sheet surface and an electroplating process for forming metallic conductor on the obtained metal coat by a continuous electroplating equipment. The invention is characterized in satisfying the (1) and (2) condition: (1) in the sputtering process, the surface resistivity of the formed metal coat is kept between 0.1 / m2 and 1.0 / m2; (2) in the electroplating process, the average cathode current density of all the electroplating bath is controlled between 1A / dm2 and 3A / dm2, and the ratio of the maximum of the cathode current density of all the electroplating bath to the minimum of that is controlled between 1 and 5, while the sheet conveying velocity is adjusted to 80-300m / h.
Owner:SUMITOMO METAL MINING CO LTD
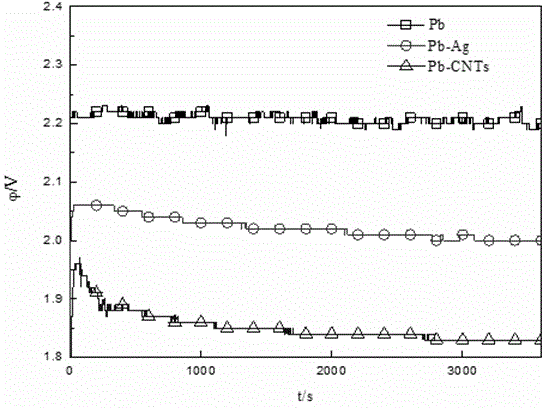
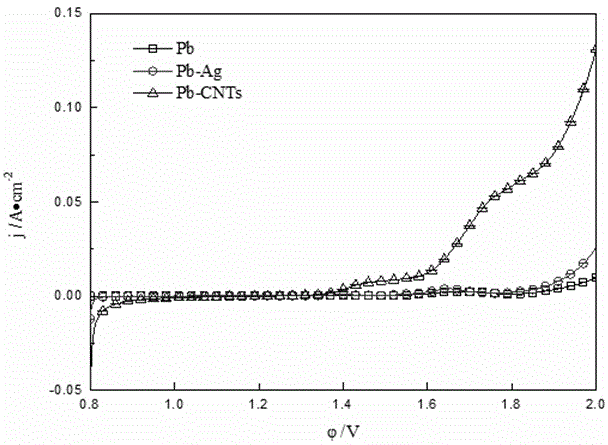
Preparation method for carbon nanotube and/or graphene reinforced lead based composite anode
Belonging to the technical field of new material preparation, the invention relates to a preparation method for a carbon nanotube and / or graphene reinforced lead based composite anode. The method includes: first, preparation of an electrolyte solution: mixing a soluble lead salt, supporting electrolyte, carbon nanotube and / or graphene, a dispersing agent and water evenly to obtain the electrolyte solution; a composite electrodeposition process: taking metal lead as the anode and adopting titanium as the cathode, controlling the electrolyte solution at a temperature of 20-80DEG C and the cathode current density at 200-500mA / cm<2>, and performing electrodeposition in the electrolyte solution so as to obtain carbon nanotube and / or graphene lead based composite powder; and preparation of the composite anode: subjecting the obtained carbon nanotube and / or graphene lead based composite powder to cold press molding at 20-50MPa, and performing sintering for 4h in a reducing atmosphere at 250DEG C, thus obtaining the composite anode. As the carbon nanotube and / or graphene have / has excellent mechanical and electrical properties, the prepared composite anode has improved mechanical properties and enhanced conductive ability.
Owner:KUNMING HENDERA SCI & TECH
Nickel electroplating solution and electroplating method thereof
The invention provides nickel electroplating solution and an electroplating method thereof. The nickel electroplating solution comprises main salt, stabilizer, complexing agent, conducting salt and reducing agent, wherein the content of the reducing agent is 10-40g / liter. The electroplating method adopting the nickel electroplating solution comprises the following steps of: firstly, putting the metal nickel into nickel electroplating solution to serve as an anode; putting a workpiece into the nickel electroplating solution to serve as a cathode; and finally, introducing a direct-current power supply, wherein the cathode current density is 0.5-5 amperes / square centimtre, the electroplating solution temperature is 40-55DEG C, and the electroplating time is 1-10 minutes. According to the invention, the technical problems existing in the traditional electronickelling and chemical nickel-plating are solved. Reducing agent is added into the electroplating solution to obviously improve the plating solution coverage capability and the corrosion-resistant capability of a nickel layer, and the electroplating solution has the advantages of strong corrosion-resistant capability and good coverage capability.
Owner:GUANGZHOU HKS SURFACE TREATMENT CO LTD
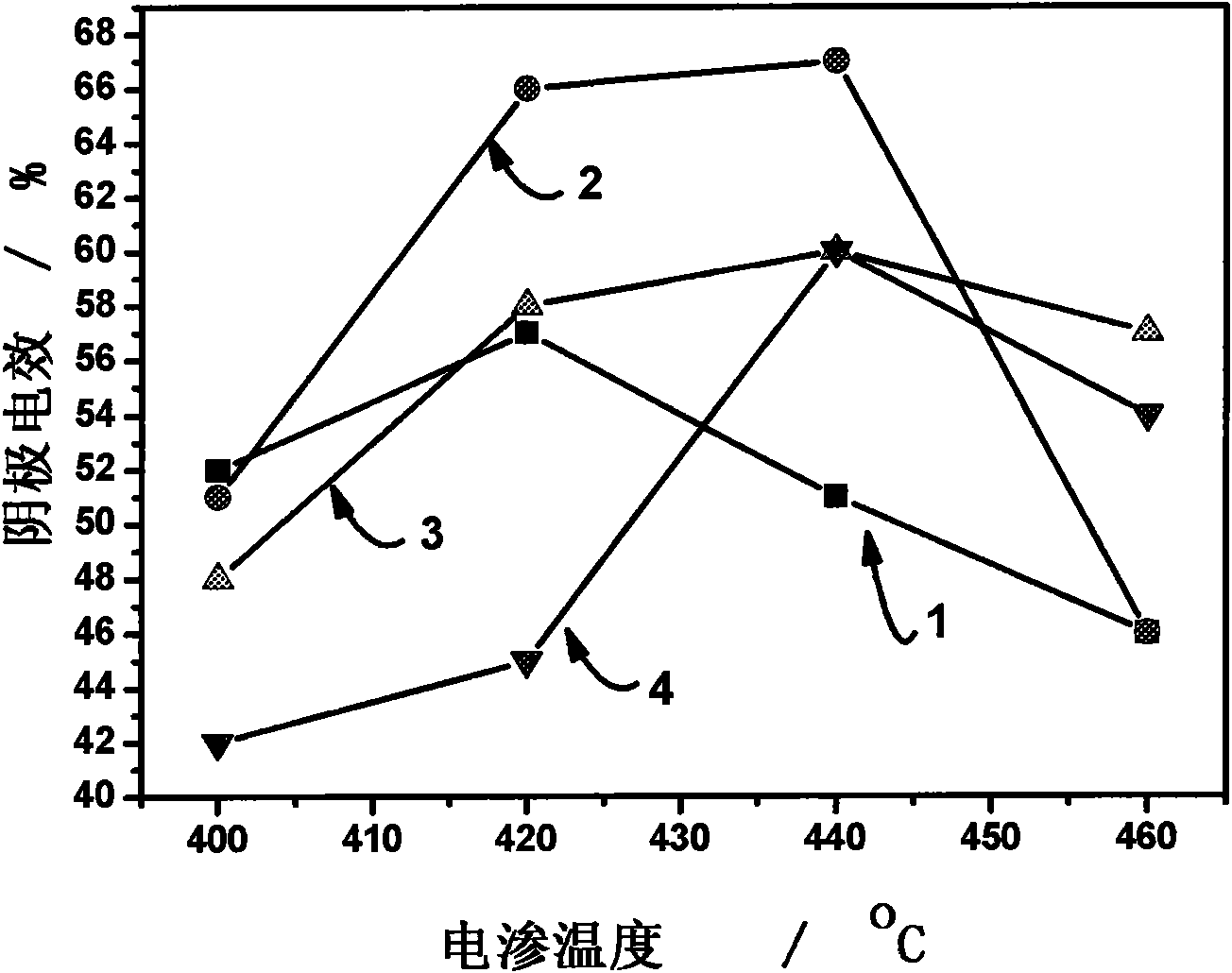
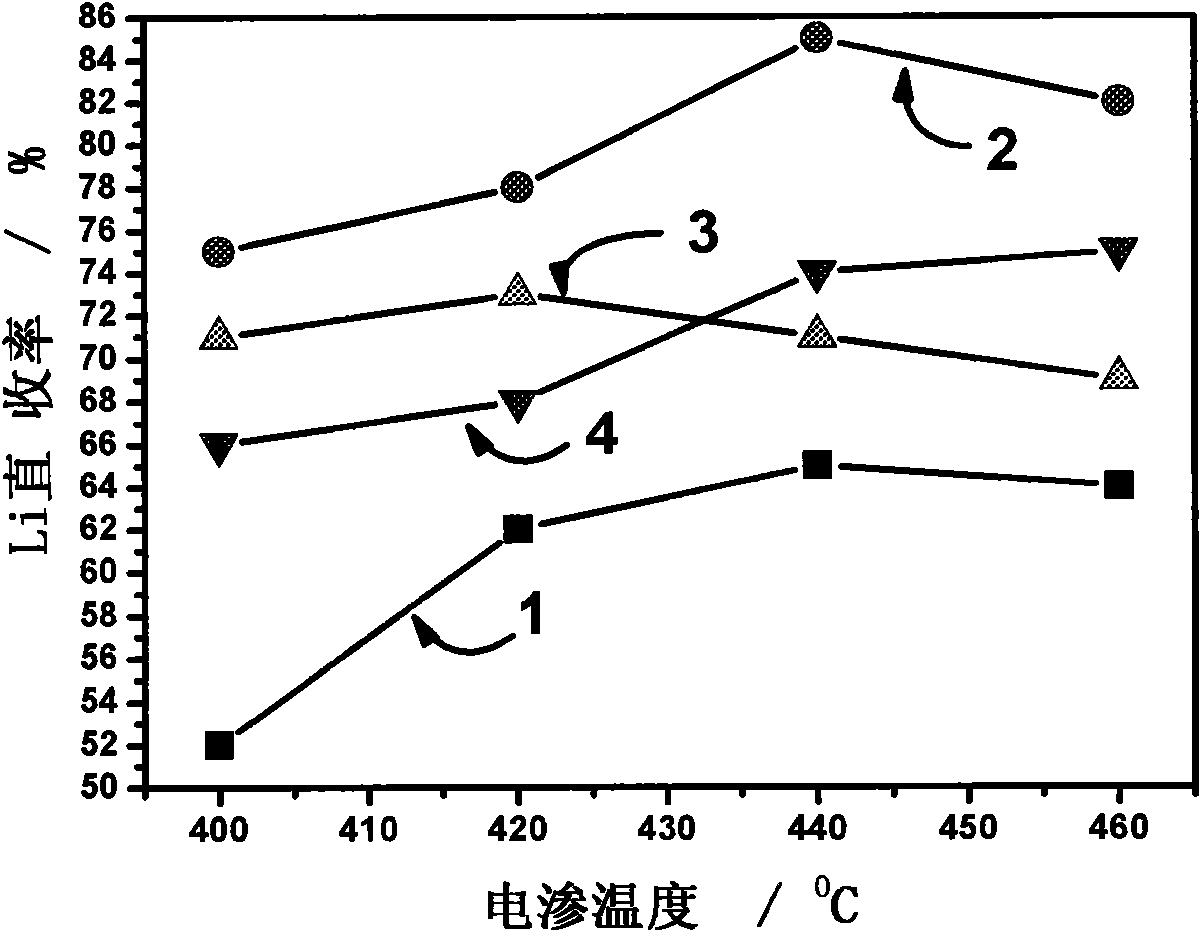
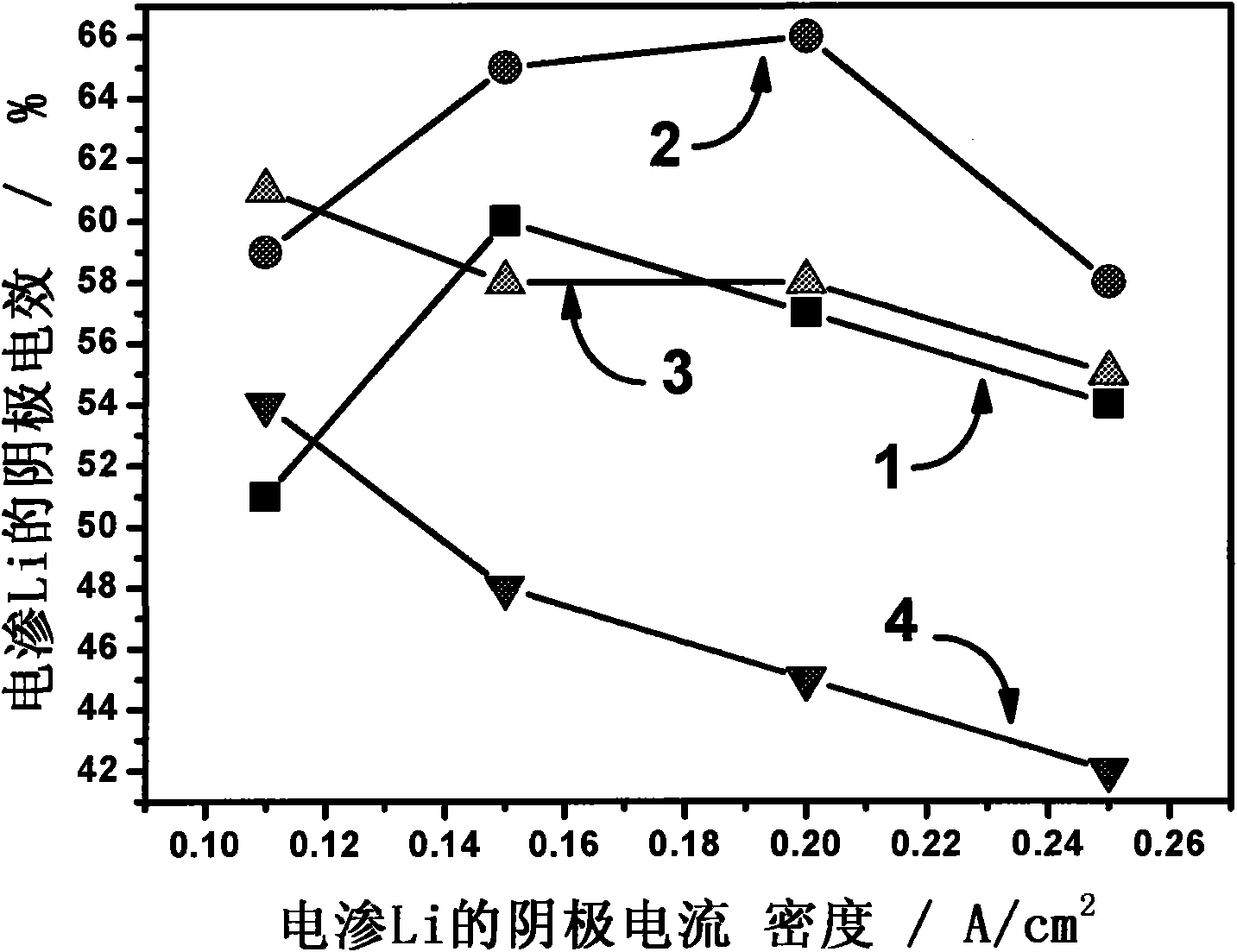
Fused salt electrosynthesis method of hydrogen storage alloy containing magnesium, lithium, sodium and potassium
The invention provides a fused salt electrosynthesis method of a hydrogen storage alloy containing magnesium, lithium, sodium and potassium, in particular to a preparation method of an electrosynthesis AB3 type hydrogen storage alloy containing magnesium, lithium, sodium and potassium, comprising a fused salt electroosmosis step and a fused salt electrolysis step which are used simultaneously. The hydrogen storage alloy containing MmNi4.2Al0.7 and the like are used as cathodes in KCl.LiCl fused salt electrolyte. The preparation method comprises the following steps of: carrying out Li electroosmosis on the cathodes, such as the hydrogen storage alloy and the like, at 420-450 DEG C; regulating the composition of the fused salt electrolyte to a quaternary mixed salt system of KCl.NaCl.LiCl.MgCl2, and also increasing the temperature; and carrying out limit electrolysis with the electrolytic cathode current density of 18-22 A / cm<2> to obtain the AB3 type hydrogen storage alloy containing magnesium, lithium, sodium and potassium. The fused salt electroosmosis step and the fused salt electrolysis step are closely linked; the Li electroosmosis is carried out at low current density, Mg is electrolyzed at high current density, and K and Na are added through compelling electrolysis so that in-situ electrodeposition is realized in a same electrolytic cell. The invention belongs to the field of a short-process touch process.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Pyrophosphate copper plating used as grounding electroplate liquid for cyanogen-free copper plating
The invention discloses a strike bath solution with pyrophosphate plating copper as the cyanide-free copper, which contains a make-up agent and rehydration salt; the make-up agent contains the following raw materials: potassium pyrophosphate, copper pyrophosphate, ammonium citrate, sorbol, sulfosalt, phenyl carboxylate, dextrin, alkyl thiourea and nitrogen heterocyclic; the rehydration salt is as supplementation of all raw materials in the make-up agent during the plating process; the invention does not contain harmful substances, such as cyanidum, heavey metal, etc and is in compliance with EU RoHS Directive (2002 / 95 / EC) with stable bath solution and wide cathode current density range, and the plating layer prepared by the invention is fine, even and in a semi-bright state; and the make-up is conducted with original solution, supplementation is conducted with single rehydration salt, which is convenient in operation and simple in management; the plating layer is well adhesive to the matrix, with good straggling capability and covering capability. The invention is applicable in pre-plating of iron materials, zinc alloys, aluminum alloys and copper alloys, as well as barrel plating and suspension plating, with the waste water easy to dispose, which will not bring the secondary pollution.
Owner:江门市瑞期精细化学工程有限公司
Chromium-free passivation solution for tin plate and method for using same
InactiveCN101638804ALow costSimple processSurface reaction electrolytic coatingChromium freeRare-earth element
The invention relates to an acid metal passivation solution and a method for using the same, in particular to a chromium-free passivation solution for a tin plate and a method for using the same. Thepassivation solution comprises the following components in portion by weight: 5 to 30 portions of inorganic salt of a rare earth element Ce or La, 5 to 40 portions of inorganic salt of ammonium and 1,000 portions of water; the pH value of the passivation solution is between 2 and 7 and can be adjusted by dilute nitric acid or dilute sulfuric acid; the inorganic salt of the rare earth element Ce orLa may be cerous nitrate, cerous sulfate, lanthanum nitrate or lanthanum sulfate; and the inorganic salt of ammonium may be ammonium nitrate or ammonium sulfate. The method for using the passivationsolution comprises that: the tin plate is soaked in the passivation solution for electrochemical processing, and during the processing, the cathodic current density is between 0.1 and 20A / dm<2>, the processing time is between 0.1 and 10s, and the temperature is between 30 and 65 DEG C.
Owner:BAOSHAN IRON & STEEL CO LTD
Electrolysis method for producing nickel foil
ActiveCN103031578AImprove yieldReduce processing costsElectroforming processesSilver plateConstant speed
The invention relates to an electrolysis method for producing a nickel foil, being applicable to the field of electrolytic production of a nickel foil. The electrolysis method for producing the nickel foil comprises the following steps of: by taking a lead-silver board or a titanium board as an anode, and a titanium roller or a stainless steel bar which is capable of rotating and controlling a rotating speed as a cathode, controlling a distance between the cathode and the anode to 9-15mm; circularly filling an electrolytic solution which consists of 200-300g / L of nickle sulfate and 40-45g / L of boric acid and is 1.7-3.5 in pH value into an electrolytic bath; turning on the power supply of the electrolytic bath and controlling a voltage, so that cathode current density is 21-35A / dm<2>; controlling the temperature of the electrolytic solution to 50-60 DEG C; rotating the titanium roller or the stainless steel bar at a constant speed, so as to peel off a nickel foil which is electrolytically deposited on the titanium roller or the stainless steel bar through the continuous rotation of the titanium roller or the stainless steel bar; and washing and drying the obtained nickel foil, and coiling the nickel foil into continuous and coiled nickel foil, to obtain the product. The nickel foil produced by the electrolytic process of the system, through the electrolytic deposition, is wide and thin, is moulded once, is comparatively high in yield and is low in processing cost.
Owner:山东雍申电子科技有限公司
Wide temperature, wide current and wide brightness type cyanide-free alkaline zinc plating process
The present invention relates to a zinc plating process method, specifically to a wide temperature, wide current and wide brightness type cyanide-free alkaline zinc plating process. The process is as follow: 1 l of a zinc plating solution comprises of 8-12 g of ZnO, 120-140 g of NaOH, 15-20 ml of a brightener, 15-20 ml of a halogenated semicarbazide polymer; the cathode current density is 0.5-8.0A / dm<2>, the operating temperature is 5-45 DEG C. According to the present invention, the plating coat prepared through the process provided by the present invention has characteristics of brightness, uniformity, low brittleness, easy passivation; the operation is easy to be controlled; the amount of the brightener consuming is reduced so as to reduce cost and pollution.
Owner:武汉吉和昌新材料股份有限公司
Method for preparing aluminum-samarium interalloy through liquid-state cathode salt fusion electrolysis method
The present invention provides a method for preparing an aluminum-samarium interalloy through fluoride fusion-electrolysis, wherein the problem of the method for preparing an aluminum-samarium interalloy through a salt fusion electrolysis method is solved. According to the method, SmF3-LiF is adopted as a salt fusion system, and Sm2O3 is added to the fused salt as an electrolyte; a tungsten rod with a diameter of 50-60 mm as a cathode, aluminum is adopted as a liquid-state cathode, and graphite is adopted as an anode; at a temperature of 750-900 DEG C and a cathode current density of 1-2 A / cm<2>, electrolysis is performed for 25-40 min, the aluminum-samarium interalloy is accumulated in the fused salt surface liquid-state cathode, the current efficiency can achieve more than or equal to 90% through combination of the partial aluminum reduction effect, and the samarium content in the aluminum-samarium interalloy can achieve more than or equal to 30%. According to the present invention, the method is practical, the problem that the divalent samarium ions and the trivalent samarium ions are subjected to reciprocated cycling so as to waste the current is effectively solved, and the interalloy of samarium is successfully obtained through the electrolysis method; the method has characteristics of high production efficiency, low fused salt inclusion, uniform composition, and extremely low production cost; and the method can further be used for preparation of other similar interalloys.
Owner:GANZHOU FEITENG LIGHT ALLOY CO
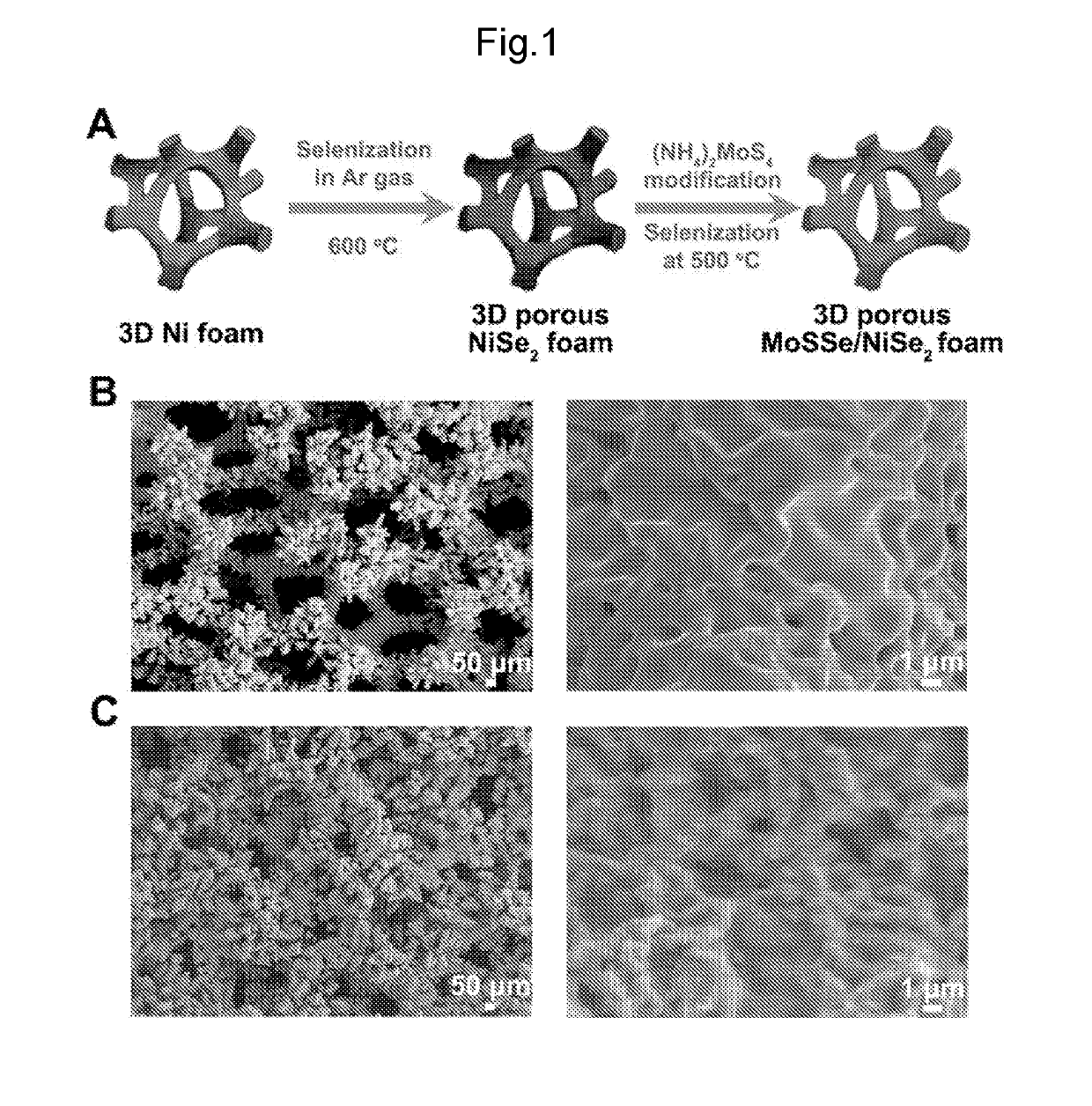
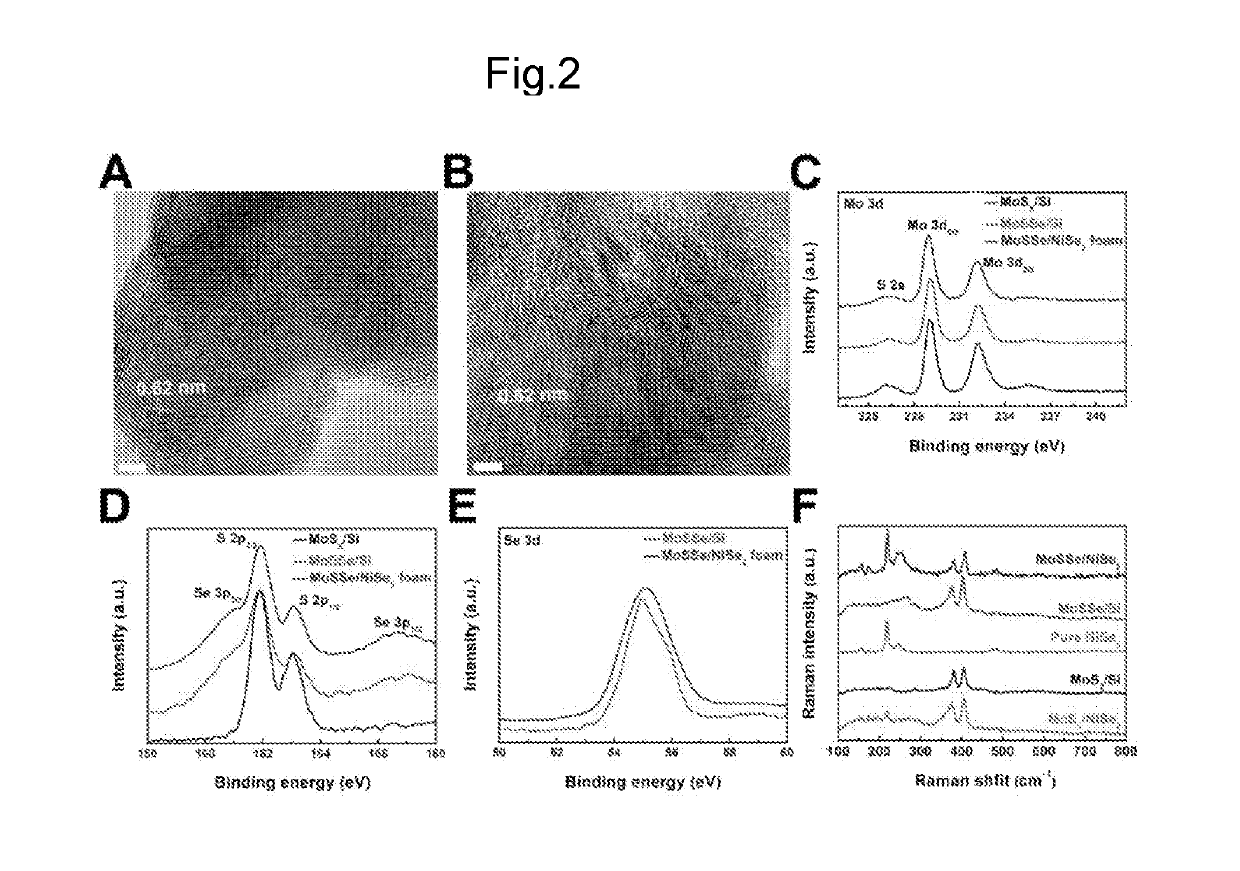
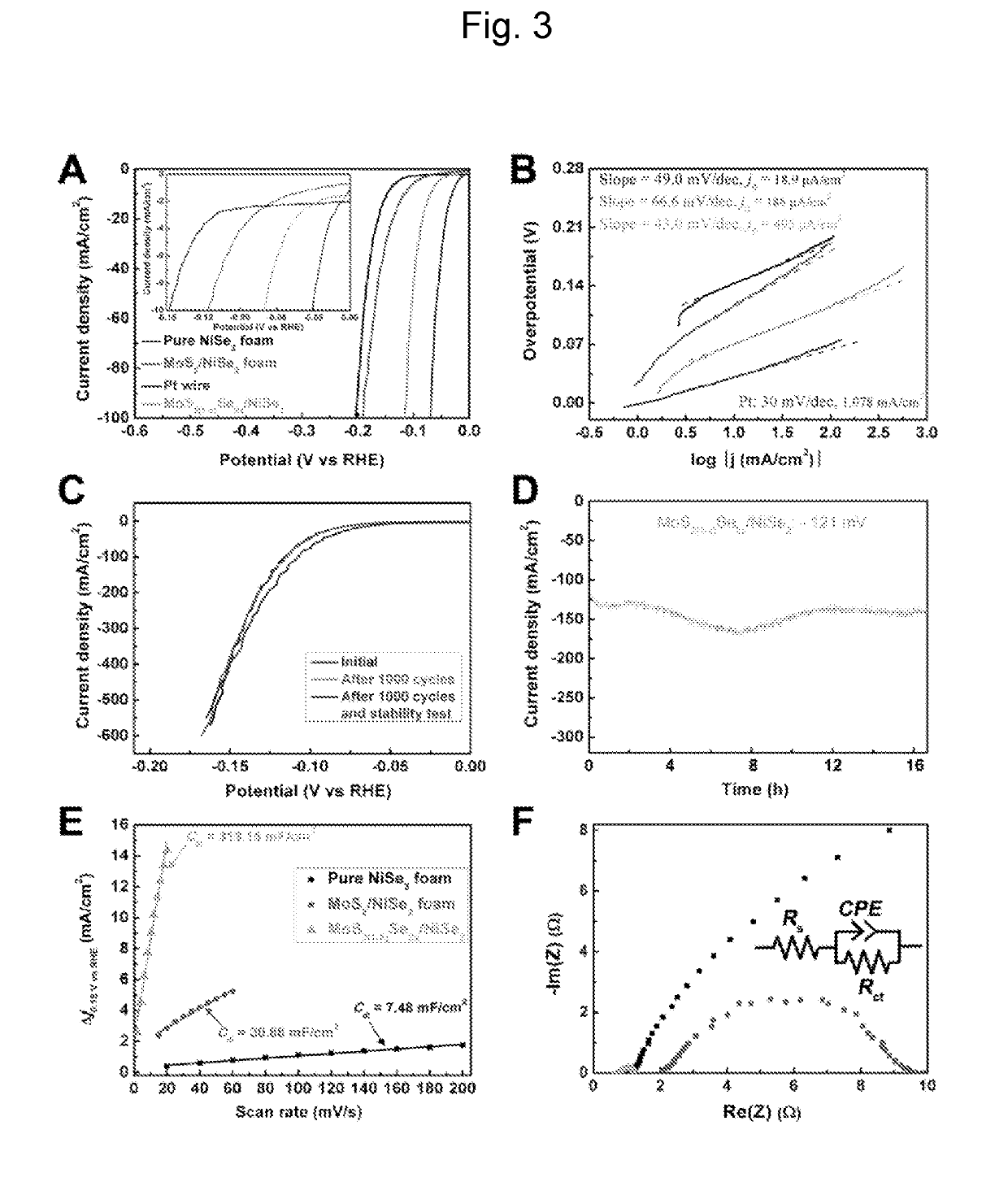
THREE-DIMENSIONAL POROUS NiSe2 FOAM-BASED HYBRID CATALYSTS FOR ULTRA-EFFICIENT HYDROGEN EVOLUTION REACTION IN WATER SPLITTING
ActiveUS20190218674A1High catalytic activityCheap and efficient and sizableCell electrodesMultiple component coatingsExchange current densityParticle position
A hybrid three dimensional (3D) hydrogen evolution reaction (HER) catalyst that is formed from a porous Ni foam support, a NiSe2 scaffold positioned on the support; and layered transition metal dichalcogenide (LTMDC) or first-row transition metal dichalcogenide particles positioned on the NiSe2 scaffold. The catalyst provides a low onset potential, large cathode current density, small Tafel slopes, and large exchange current densities, similar in catalytic power to Pt HER catalysts.
Owner:UNIV HOUSTON SYST
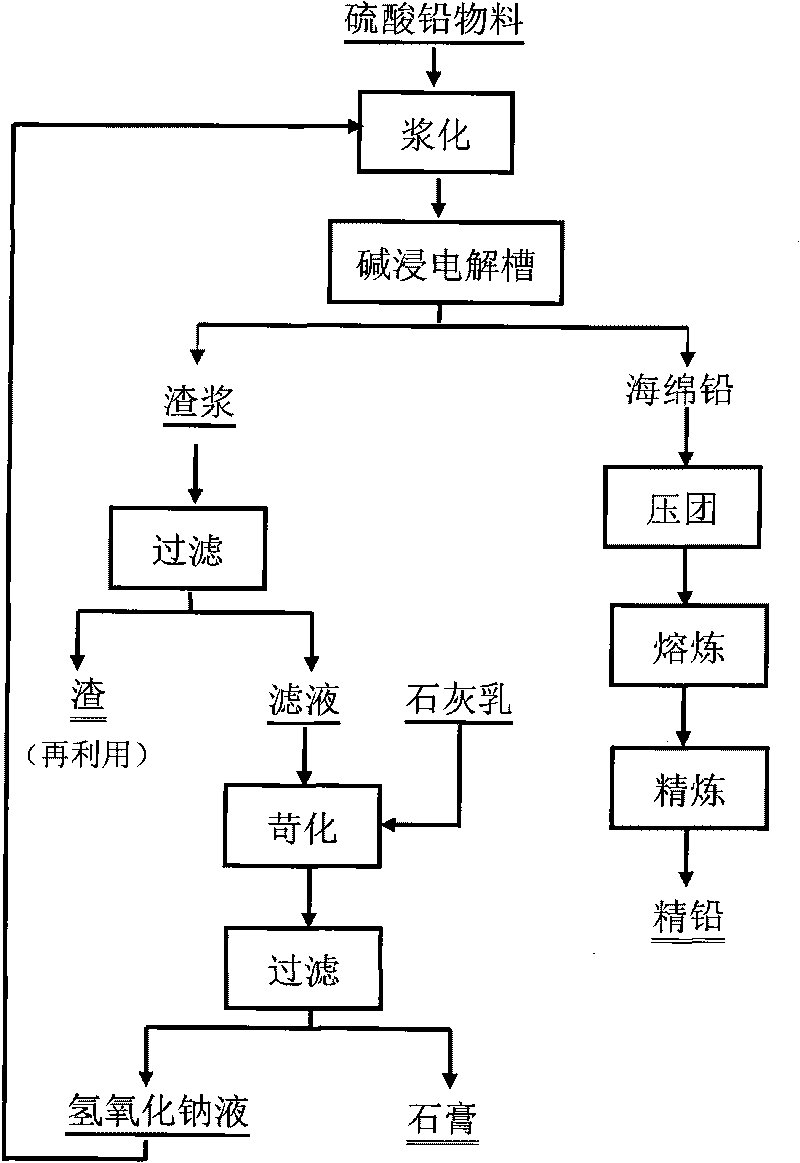
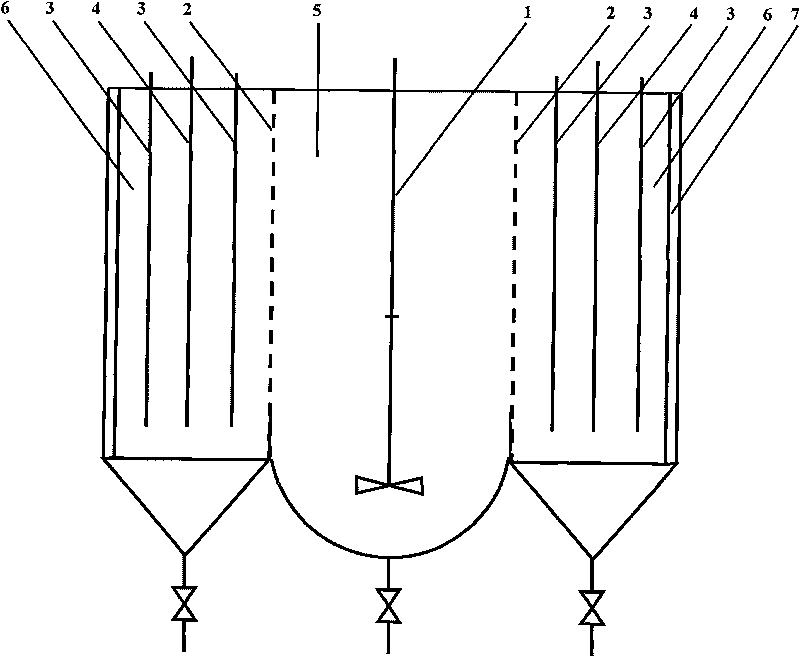
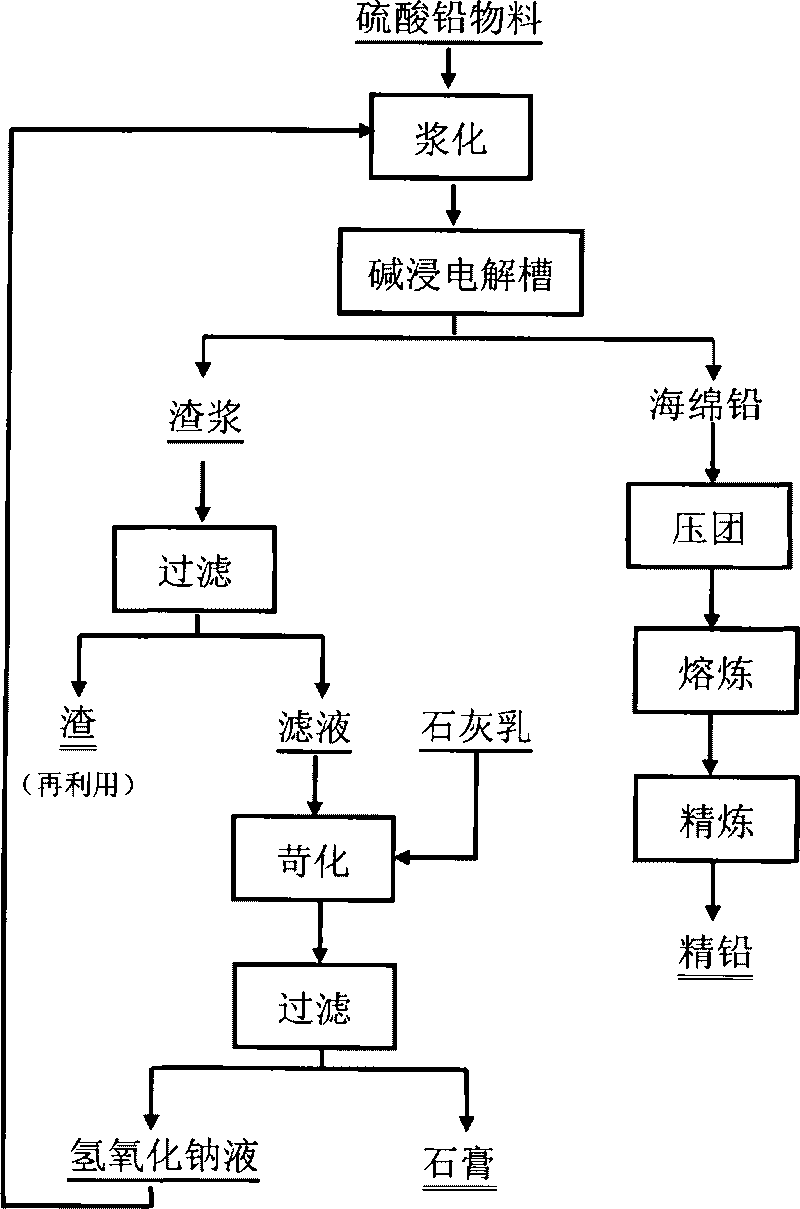
Method for producing lead by executing electrolysis and alkaline leaching on lead sulfate material
ActiveCN101760757AWithout difficultyHigh recovery ratePhotography auxillary processesProcess efficiency improvementElectricitySlurry
The invention relates to a method for producing lead by executing electrolysis and alkaline leaching on lead sulfate material, comprising the following steps: placing the pasted lead sulfate material to a blending infiltration chamber of an alkaline leaching electrolysis bath, introducing DC electricity to the electrolysis bath, dissolving lead in the lead sulfate by sodium hydroxide so as to enter the solution, passing through a partition film to enter an electrolysis chamber, enabling lead ions in electrolyte to discharge and separate out on a cathode, obtaining metallic sponge lead or lead particles, regenerating partial sodium hydroxide in the solution, leading the electrolyte of the electrolysis outside, returning the electrolyte to the blending infiltration chamber to recycle after causticization treatment. The main alkaline leaching conditions are as follows: the cathode current density is 200-1000A / m2; the electrolyte temperature is 20-90 DEG C; the groove voltage is 1-5V; the electrode homopolar distance is 50-120mm; and the liquid and solid ratio of the slurry is 5-12: 1. The lead sulfate alkaline leaching and the lead electrolysis are carried out simultaneously in a same device, thereby regenerating solvent sodium hydroxide, simplifying the working procedures, reinforcing the dissolving process of the lead-bearing material, and widening the application range of the method on the lead-bearing material.
Owner:GREENNOVO ENVIRONMENTAL TECH CO LTD
Method for electrodepositing zinc and zinc alloy at low temperature by ionic liquor
InactiveCN102517608AImprove solubilityImprove conductivityPhotography auxillary processesProcess efficiency improvementDecompositionHeat stability
The invention discloses a method for electrodeposit zinc and zinc alloy at low temperature by ionic liquor, which includes the following steps of 1) dissolving zinc chloride ZnCl2, or zinc chloride ZnCl2 and alloying element chloride MeCln into mixed ionic liquor to prepare electrolyte; 2) utilizing high-purity graphite as an anode and solid zinc or metallic copper as a cathode to realize direct-current electrodeposition; 3) arranging the anode and the cathode into an electrodepositing tank vertically; 4) controlling constant potential, cathode-current density, electrode distance and electric depositing temperature; and 5) generating chlorine gas at the anode and separating solid zinc from the cathode. During electrodeposition, the voltage of the electrodepositing tank is higher than higher decomposition voltage in zinc chloride or alloying element chloride while lower than that of an electric chemical window of the ionic liquor, zinc or zinc alloy is produced on the cathode, and the chlorine gas is discharged from the anode. The ionic liquor is light in weight, non-toxic, excellent in conductivity, high in heat stability, low in melting point, high in boiling point, wide in the electric chemical window and capable of alleviating corrosion to the electrode materials and tank liners, can be recycled and has no volatility or flammability.
Owner:IRICO
Method for preparing rare-earth dysprosium alloy by molten salt electrolysis
InactiveCN103924265AImprove ingredient consistencyStrong distribution controllabilityTin FluoridesRare earth
The invention relates to a method for preparing a rare-earth dysprosium alloy by molten salt electrolysis. The method is characterized by comprising the following steps: adding a mixture of a rare earth oxide and a dysprosium oxide as an electrolytic material in a fluoride molten salt system to carry out electrolysis production, wherein the fluoride molten salt system adopts a ternary system of 90-80% of rare earth fluoride, 7-10% of dysprosium fluoride and 3-10% of lithium fluoride by weight percent; the weight percent ratio of the rare earth oxide to the dysprosium oxide in the raw material mixture is (95-80):(5-20); and the electrolyzing temperature is 1030+ / -30 DEG C; carrying out electrolysis by adopting a plug or bottom cathode method when the cathode current density is 6.0+ / -2.0A / cm<2>, the anode current density is 1.3+ / -1.0A / cm<2> and the cell voltage is 5-10V; and depositing the rare-earth dysprosium alloy near the cathode. The method has the advantages that the problems of complicated process, serious environmental pollution, high production cost and the like of the related technique at present are effectively overcome, the prepared alloy ingredient is even, and the method is suitable for production of a large electrolytic cell of over 10,000A.
Owner:NAT ENG RES CENT OF RARE EARTH METALLURGY & FUNCTION MATERIALS
Method for performing continuous codeposition on Al-Mn alloy plating layer in molten salt system
InactiveCN102061490ASave heatReduce oxidation lossElectrolysis componentsRare-earth elementVoltage control
The invention relates to a method for performing continuous codeposition on an Al-Mn alloy plating layer in a molten salt system. A metal material (a steel plate) is used as a cathode and a pure aluminum material with the purity of over 99 percent is used as an anode. The method comprises the following steps of: adding a mixture of MnCl2, RClX, AlCl3, NaCl and KCl into an electrolytic bath; heating and melting; electrolyzing electrolytes, wherein R is a rare earth element, the electrolysis temperature is between 160 and 220 DEG C, the cathode-current density is controlled to be between 50 and 100 mA / cm<2>, and the bath voltage is controlled to be between 1.9 and 2.3V; stirring in the electrolytic bath, wherein the electroplating time is between 20 and 30 minutes; and plating for the second time and the third time after finishing plating for the first time, wherein the whole process is performed under the protection of argon. By the method, an Al-Mn plated alloy is directly obtained by one step through molten salt electrolysis and codeposition, and the appearance quality and the corrosion resistance of the plating layer after continuously plating under the environment can remain consistent basically.
Owner:淄博德丰化工有限公司
Alkali system electroplating bright zinc-nickel alloy process
The invention relates to a process for electroplating zinc-nickel alloy, in particular to an alkali system electroplating bright zinc-nickel alloy process. The formula of electroplating liquid is that: one liter of electroplating liquid comprises 8 to 12 grams of ZnO, 100 to 140 grams of NaOH, 45 to 60 milliliters of complexing agent, 12 to 18 grams of NiSO4.6H2O, 35 to 45 grams of C6H6O5 and 8 to 12 milliliters of brightening agent. The zinc-nickel alloy is electroplated at room temperature, the cathode-current density is 0.5 to 5.0 A / dm<2>, and the electroplating time is 15 to 30 minutes. The coating obtained by the process is bright, uniform, low in fragility and easy in passivation; the content of nickel in the alloy coating is stable and between 10 and 14 percent; influence of the temperature and the cathode-current density is little; and the process is easy to operate and control.
Owner:武汉吉和昌新材料股份有限公司
Method of analyzing electrolytic copper plating solution, and analyzing device therefor and production method for semi-conductor product
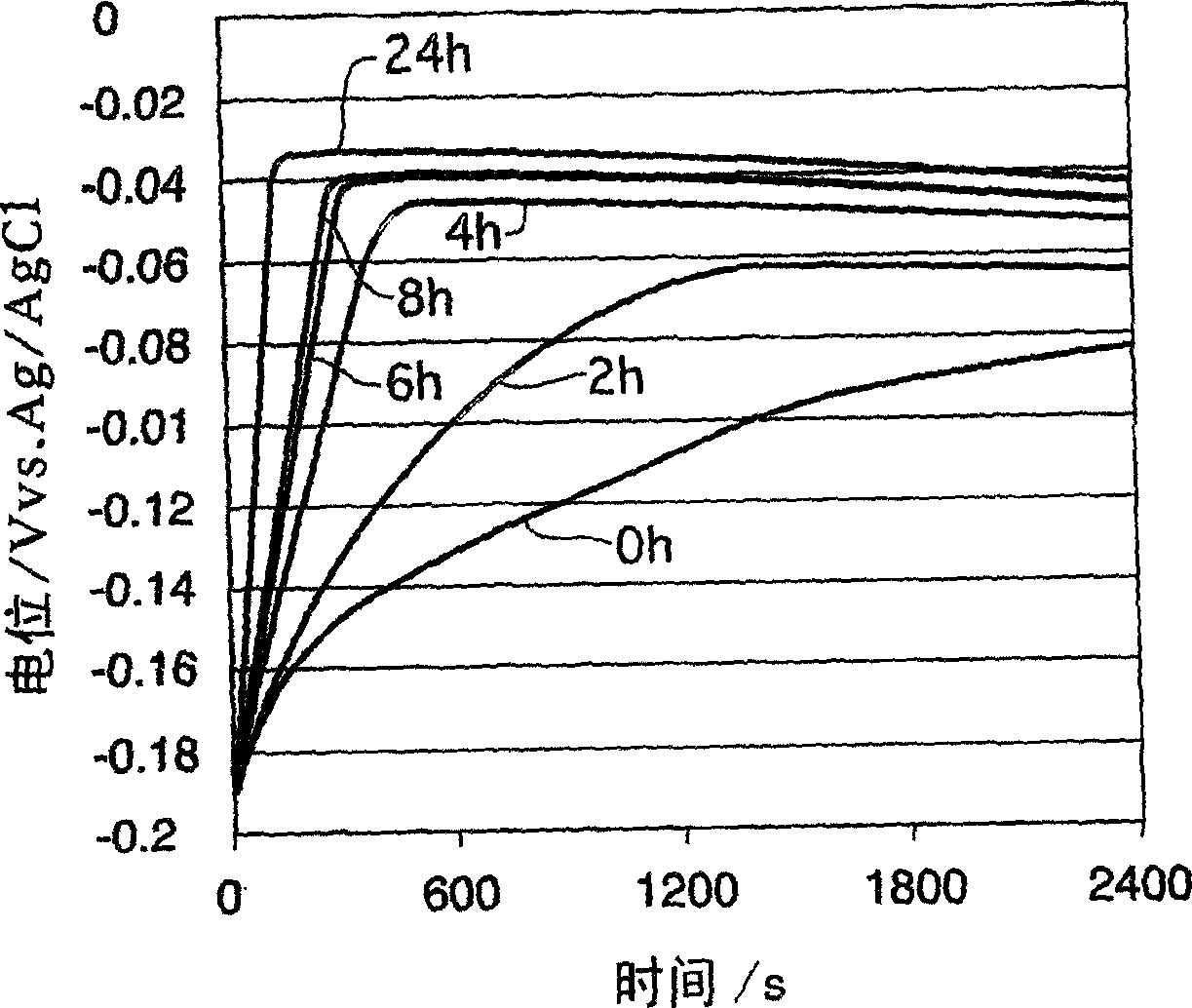
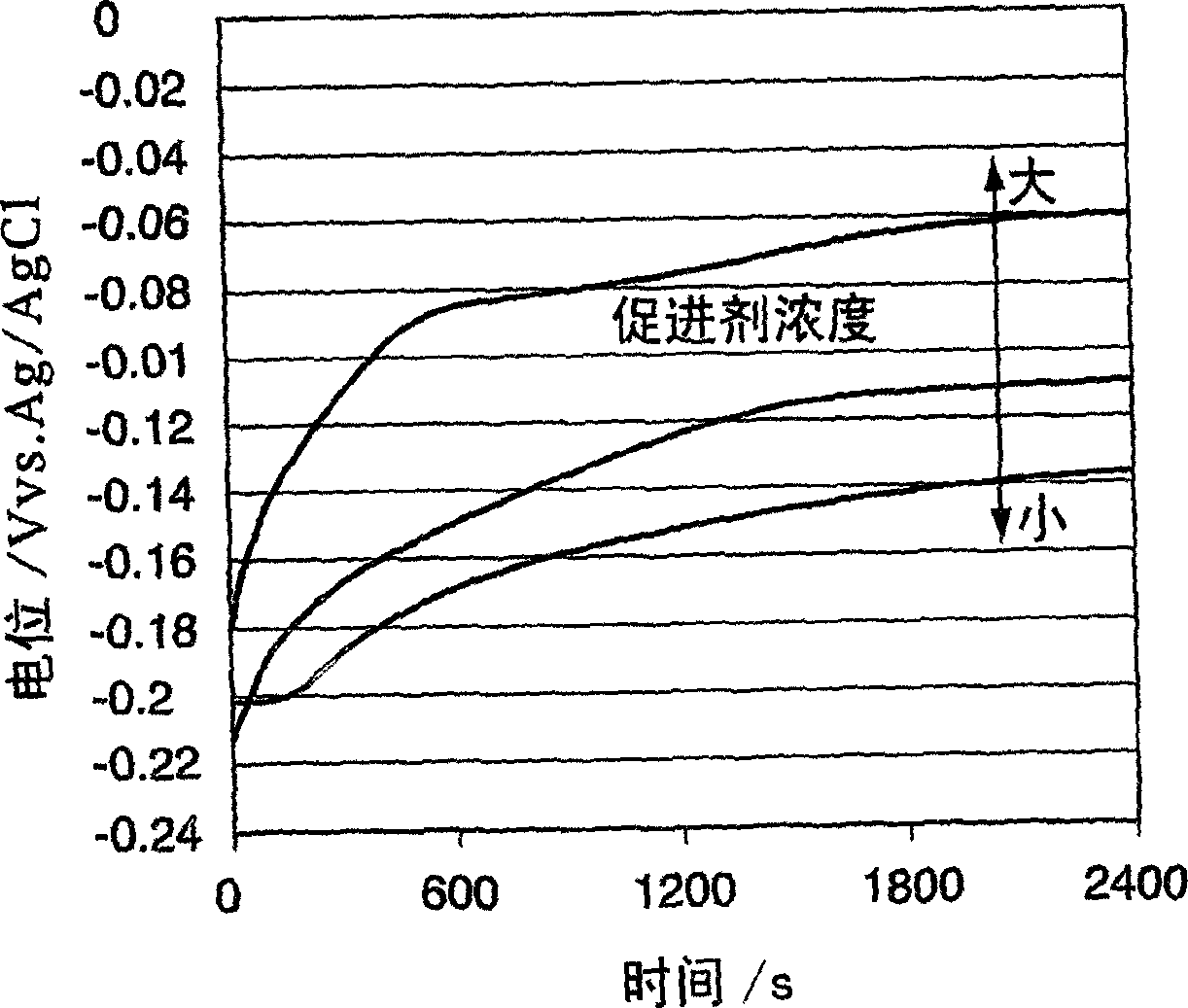
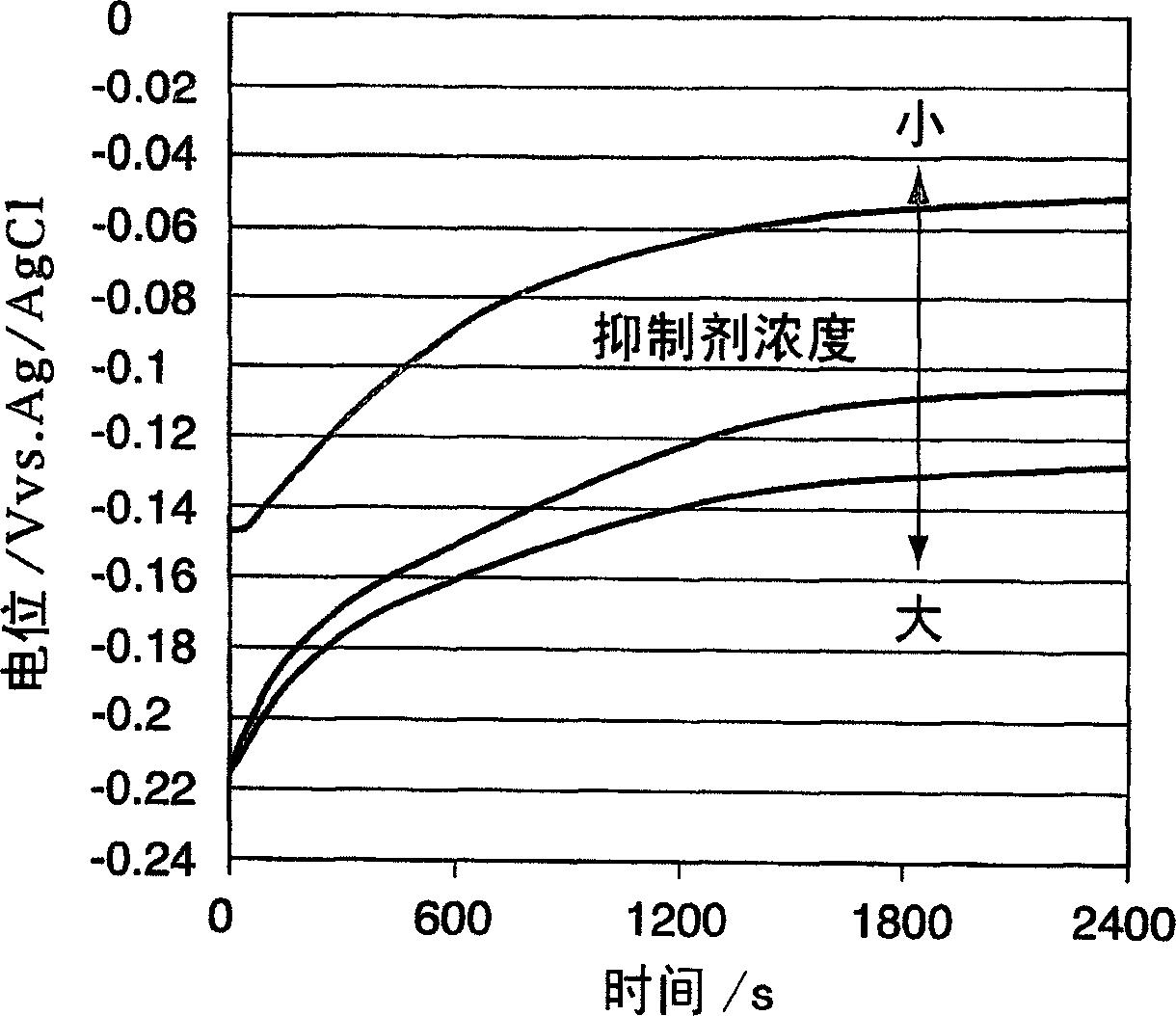
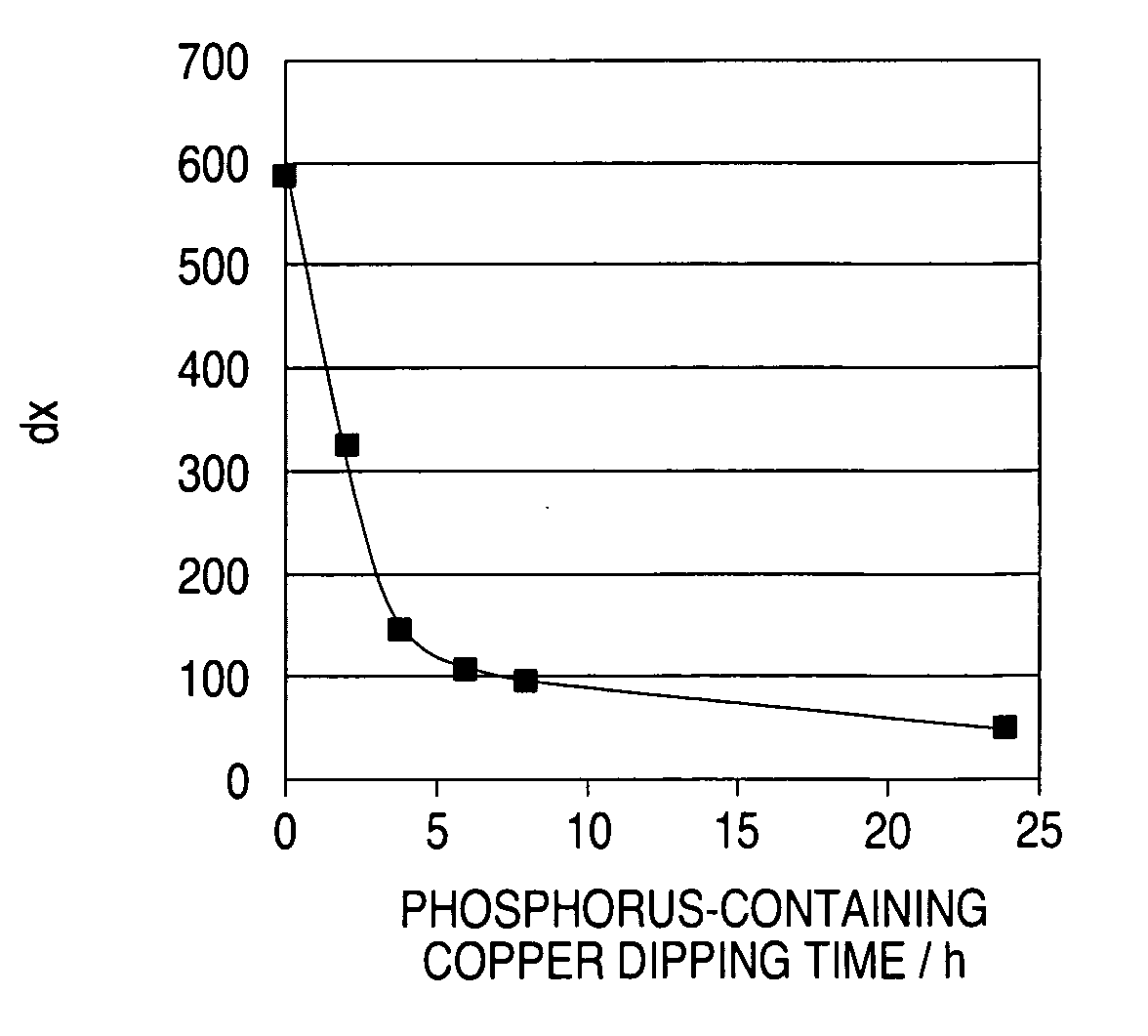
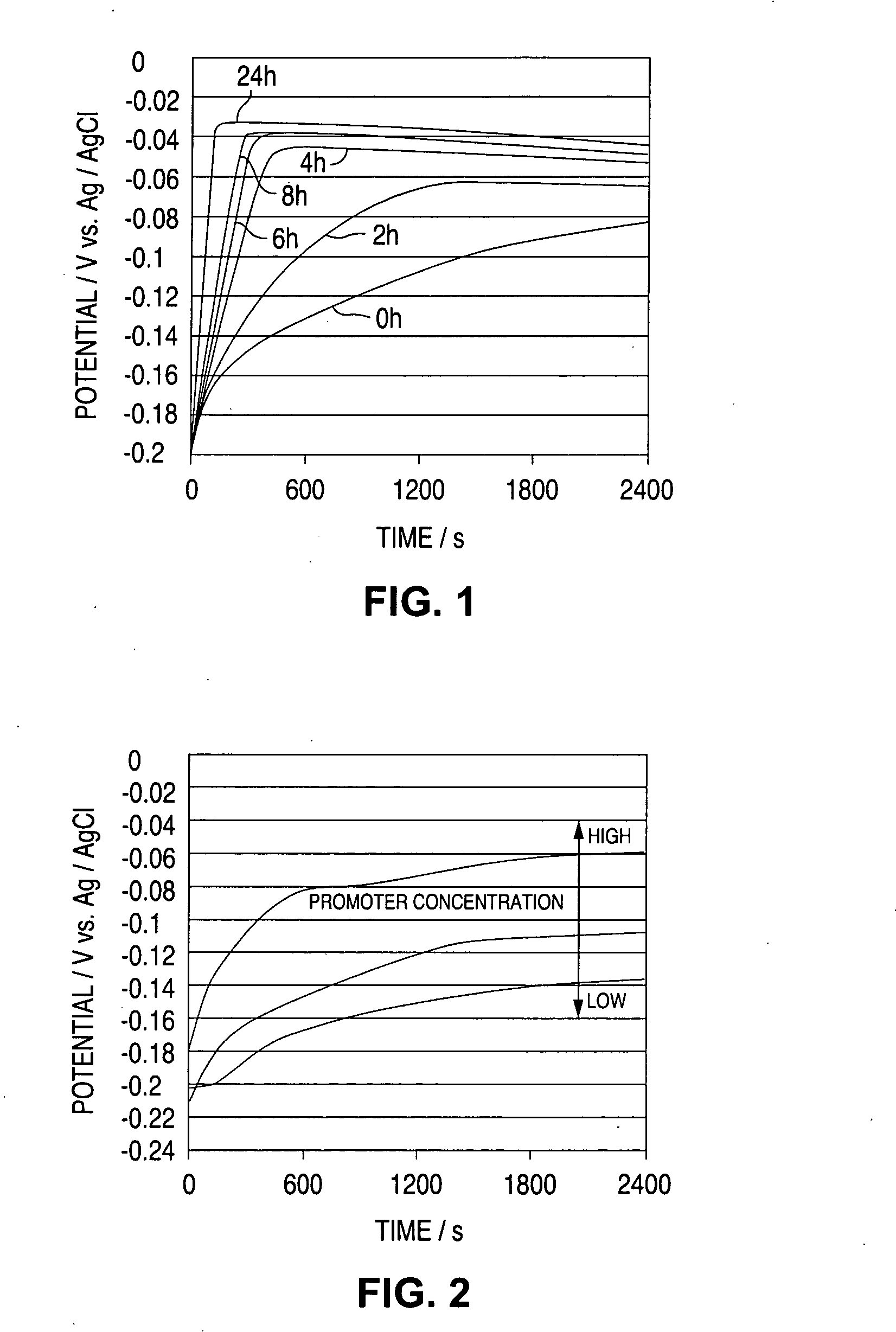
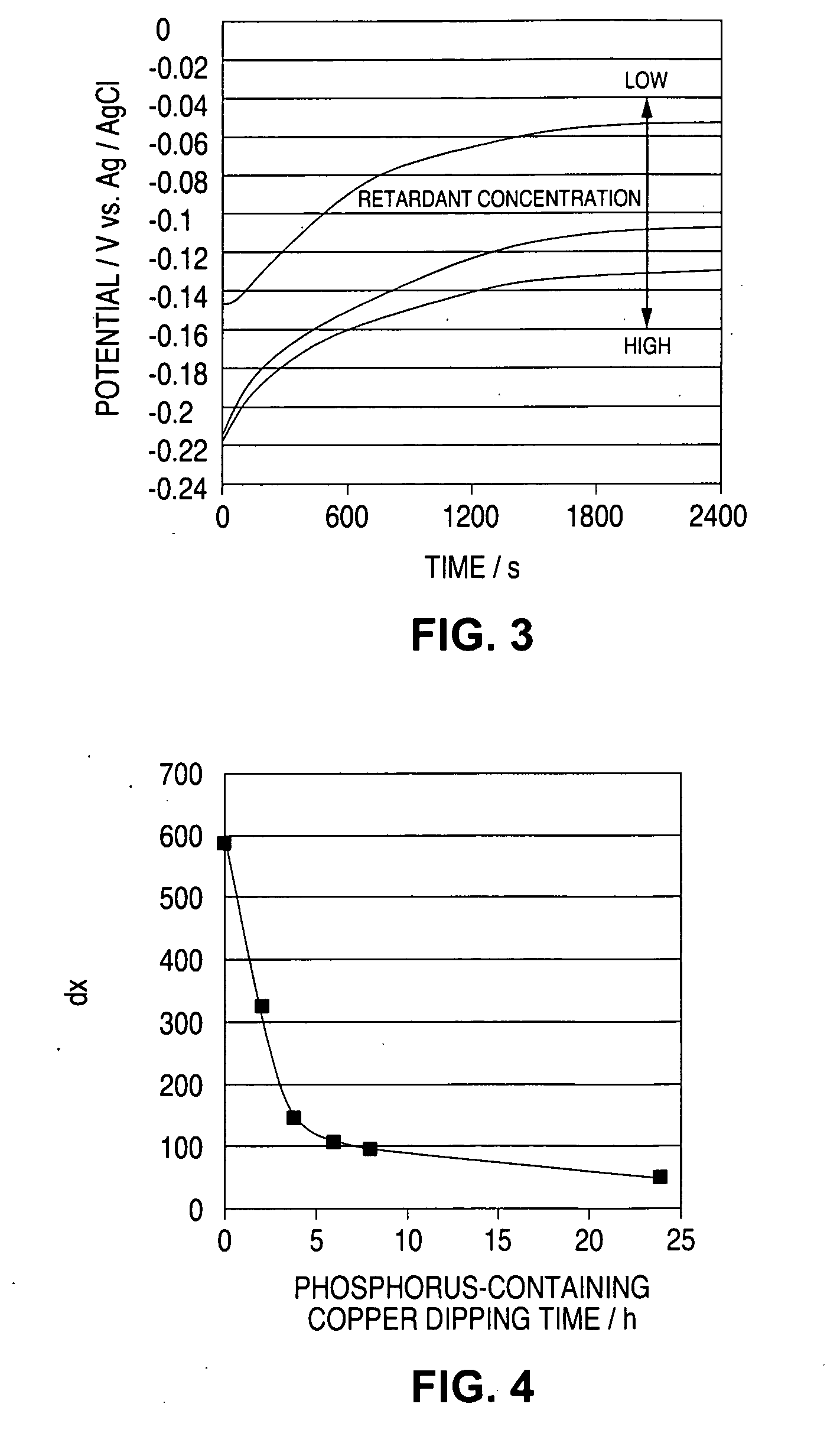
A time-dependent change in potential at a cathode current density of 0.1-20 A / dm<2> is measured to thereby judge the effective embeddability and uniform electrodepositing property of an electrolytic copper plating solution. The measurement is carried out with the rotation speed of a working electrode set at 100-7500 r.m.p., and embeddability is judged from its curved shape. A curve of time-dependent change in potential for a specified time after starting electrolysis is approximated by Boltzman function, and a potential change speed dx and a potential convergent point A2 are determined to judge embeddability.
Owner:TOPPAN PRINTING CO LTD
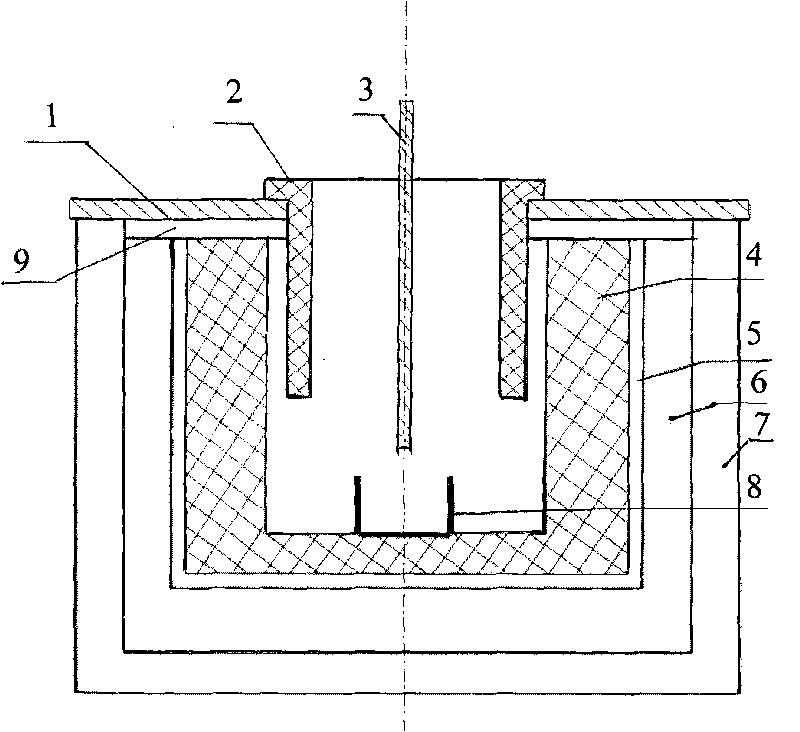
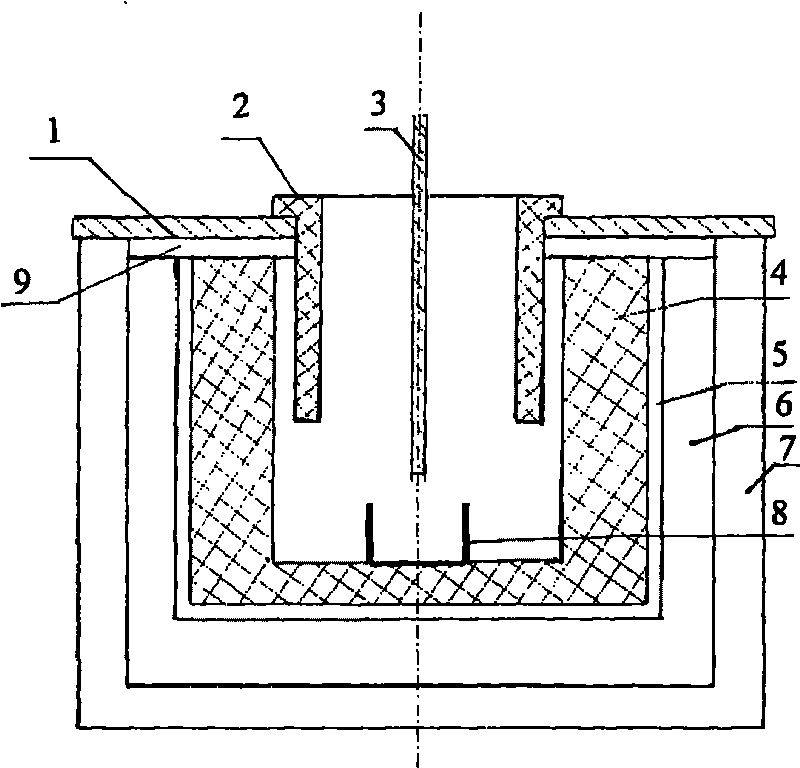
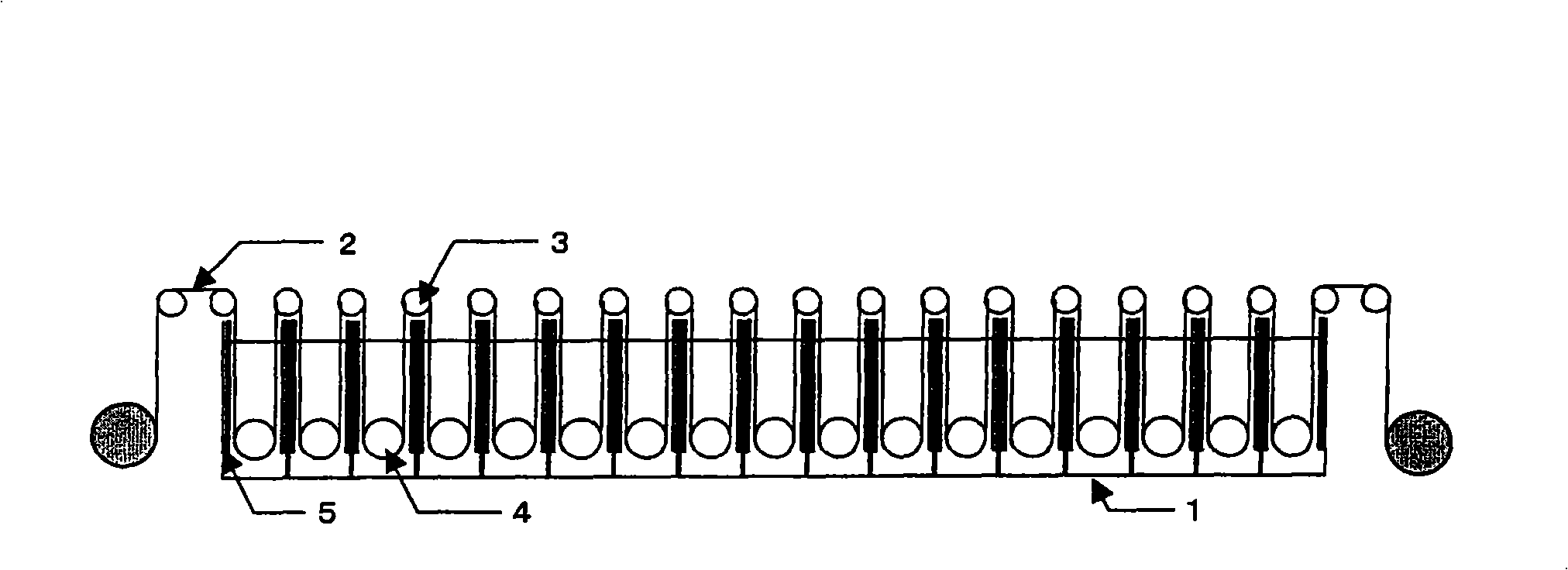
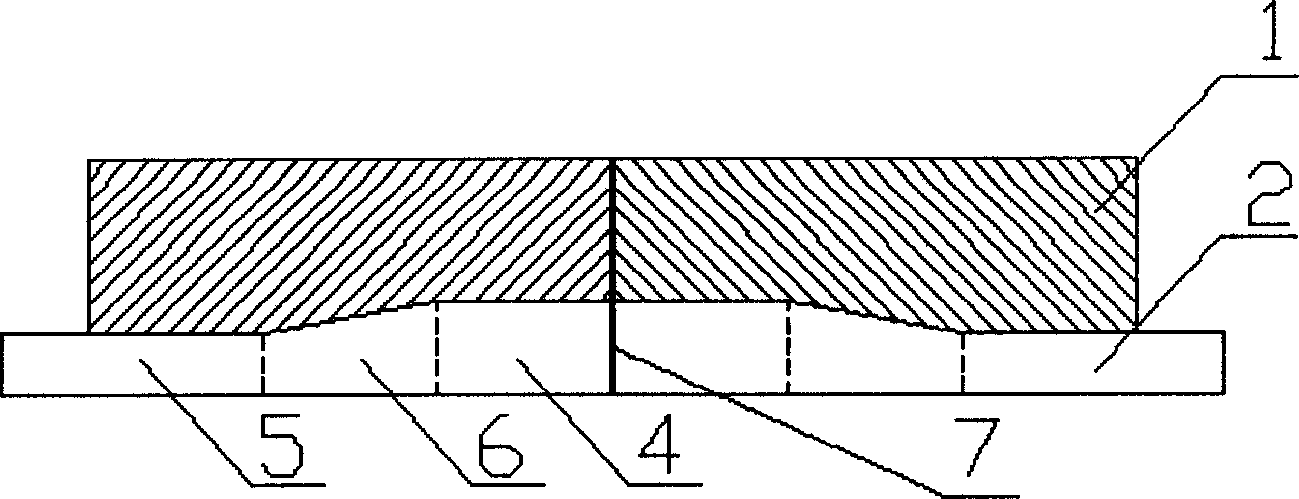
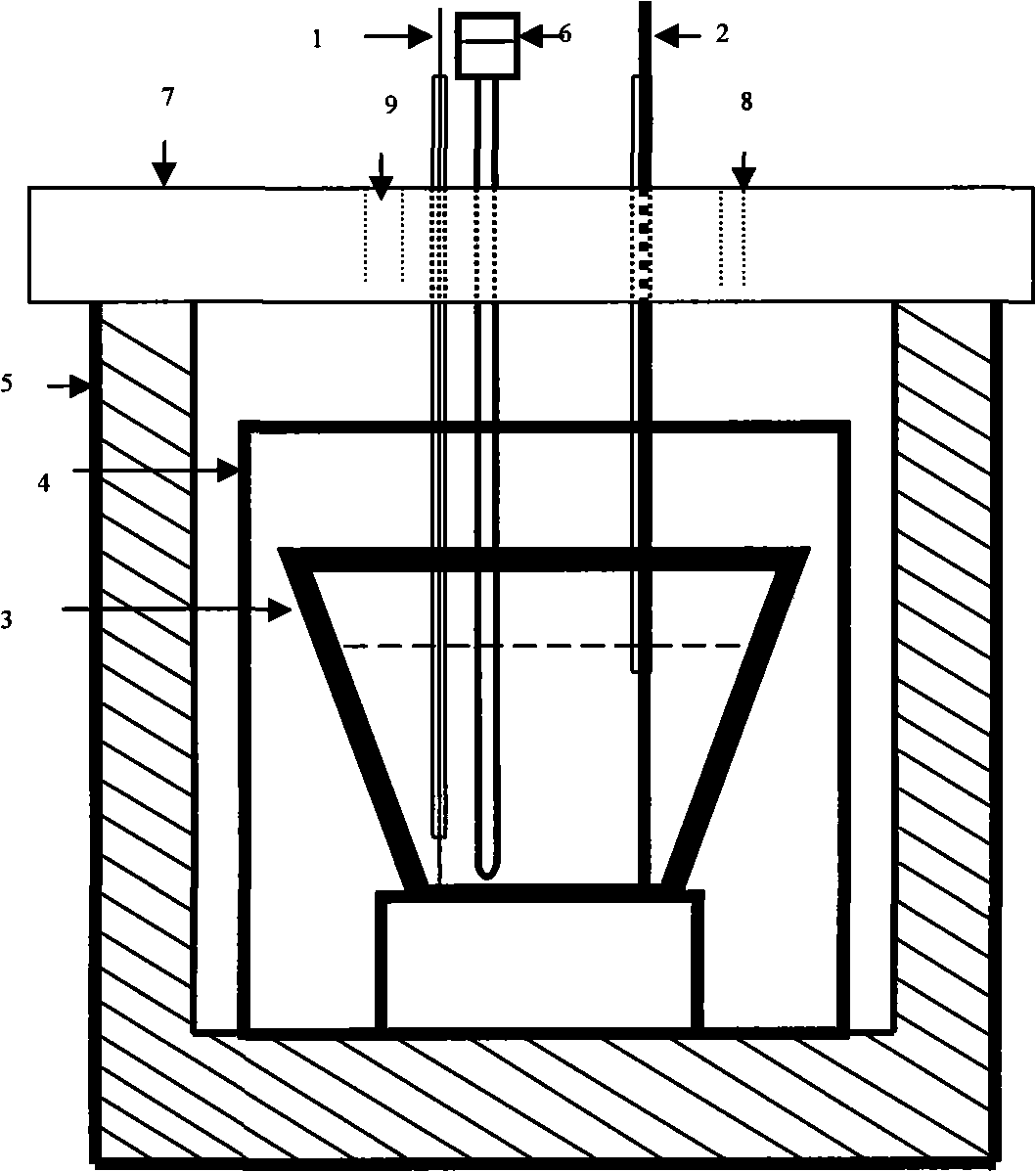
Method and structure for improving cathode current density of aluminium-electrolytic cell
ActiveCN1844467AImprove stabilityImproved current density distributionElectrical resistance and conductanceSteel bar
This invention discloses an aluminum electrolytic trough cathode current density method and structure, wherein the aluminum electrolytic trough is embed with cathode bottom block with steel bar, when embedding the steel bar into the cathode bottom block, making the steel bar into the shape whose middle section is large, two ends section is small, and the space between large section and small section is used a slope variable cross-section to connect, then embedding the steel bar in cathode bottom block, which can make the resistance at the electrolytic trough middle and border incline to consistent to improve the current disposition of the cathode bottom block and reduce the electrolytic corrosion of the cathode bottom block.
Owner:GUIYANG AL-MG DESIGN & RES INST
Method for analyzing electrolytic copper plating solution, and analyzing device therefor and production method for semi-conductor product
InactiveUS20060183257A1Satisfactory fillabilityCellsSemiconductor/solid-state device testing/measurementCopper platingPotential change
Effective fillability and the uniformity electrodeposition of a copper electroplating solution is judged by determining the time-dependent potential change thereof at a cathode current density of 0.1-20 A / dm2. The potential change is determined at a working electrode rotation of 100-7500 rpm, and the fillability with the solution is judged from the curve profile. The time-dependent potential change curve within a predetermined period of time after the start of electrolysis is approximated according to the Boltzmann's function, and the potential change speed dx and the potential convergent point A2 are obtained to judge the fillability with a plating solution.
Owner:TOPPAN PRINTING CO LTD
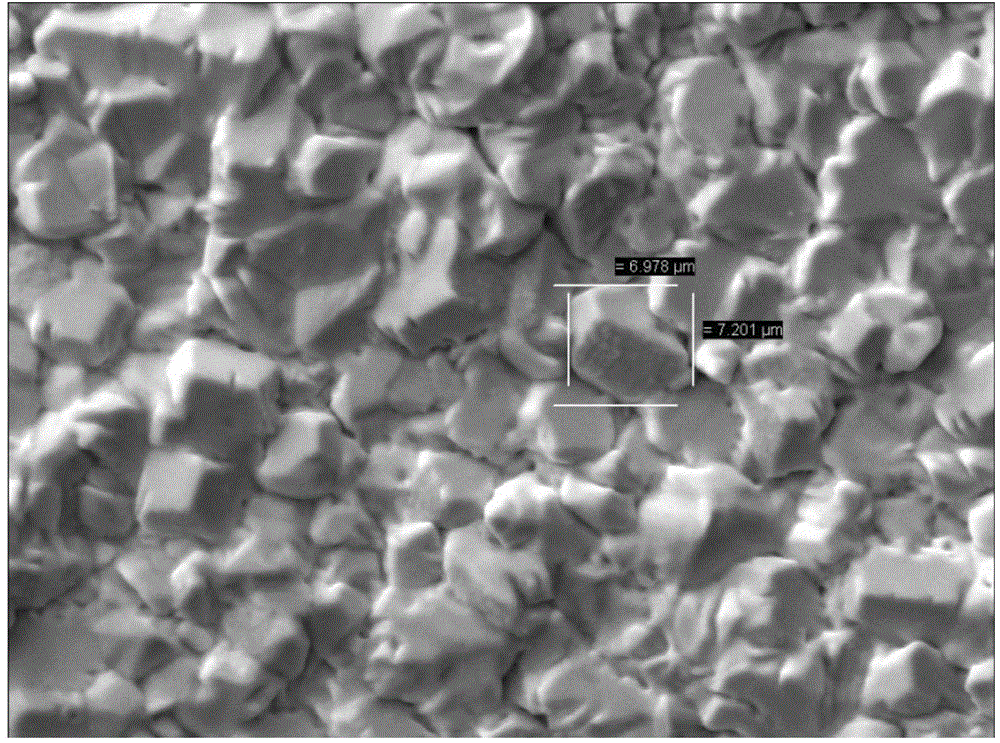
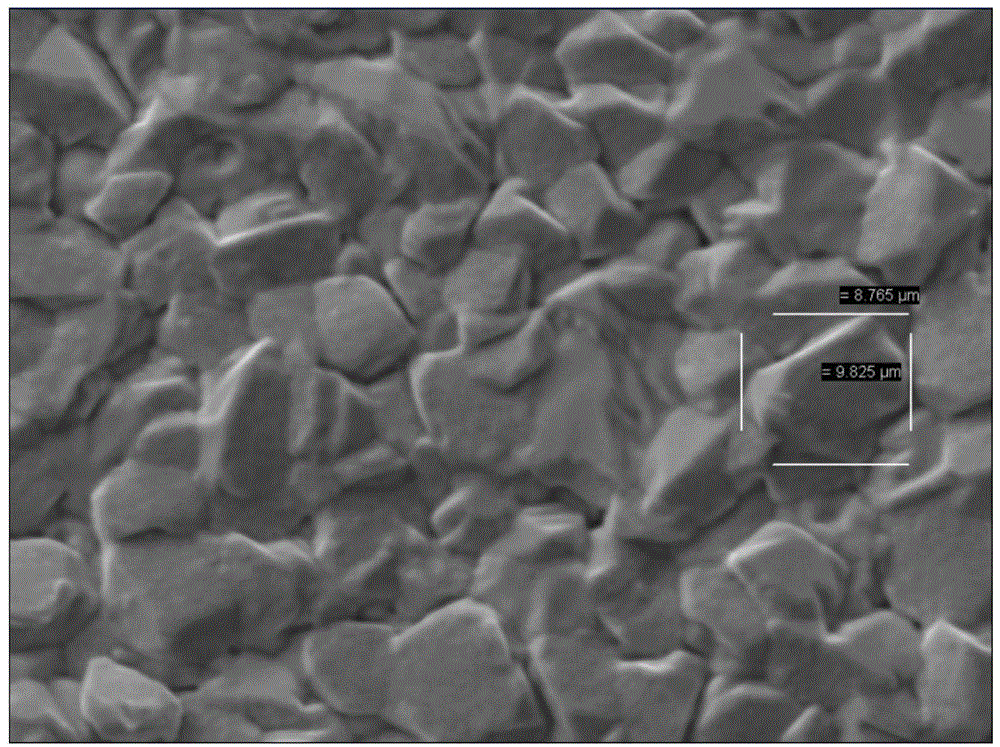
Alloy nanotube and manufacturing method thereof
InactiveCN101469453AEasy to manufactureThe preparation process is easy to controlPolycrystalline material growthSingle crystal growth detailsNanoholeNanotube
The invention discloses an alloy nano-tube and a preparation method thereof, which belong to the field of nanometer material. The alloy nano-tube is a solid solution, a metal compound or an amorphous state formed by two transition metal elements, namely A and B, wherein the A accounts for 15.3 to 86.4 percent of the total amount, and the B accounts for 13.6 to 84.7 percent of the total amount. The preparation method comprises the following steps: a formwork with a layer of gold or platinum film introduced into the back side is soaked in an electrolysis bath containing metal ion A and B, and is electrodeposited under certain voltage and current conditions to obtain a binary alloy nano-tube. Binary alloy nano-tubes with different diameters, different lengths and different components can be obtained by adopting formworks with nanochannels with different inner diameters, controlling the electrodeposition time and adjusting cathode current density. The binary alloy nano-tube has simple preparation process, mild reaction conditions, strong controllability and good application prospect.
Owner:BEIJING UNIV OF CHEM TECH
Tin-zinc alloy electroplating method
The invention aims to provide a tin-zinc alloy electroplating method. The method is performed under the following conditions: plating bath temperature: 15-25 DEG C; plating solution stirring speed: 50-200m / min; and cathodic current density: 5-100A / dm2. The tin-zinc alloy electroplating method is obtained by selection of a plating bath temperature, a cathode current density and the like and the use of a specific tin-zinc alloy plating bath, and the tin-zinc alloy plating obtained using the method has strong corrosion resistance and reduced pollution, and is an ideal substitute for cadmium plating.
Owner:东莞市闻誉实业有限公司
Mg-Li-Ho alloy, and fused salt electrolysis preparation and apparatus thereof
The invention provides a Mg-Li-Ho alloy and a method for preparing the same by molten salt electrolysis. The method comprises the following steps of: adding Ho2O3 in an electrolytic furnace by taking MgCl2 plus LiCl plus KCl plus KF as an electrolyte system, and heating up the mixed electrolyte system to fuse at a temperature of 650 DEG C; depositing the Mg-Li-Ho alloy in a molten salt bath close to a cathode via electrolyzing for 1 to 2 hours with a sunk cathode method with metal molybdenum (Mo) as the cathode, graphite as an anode, cathode current density between 12 and 16 A / cm<2>, anode current density between 0.5 and 0.6 A / cm<2> and bath voltage between 4.6 and 7.6V. The method completely uses metal compounds as raw materials to directly prepare the Mg-Li-Ho alloy through molten salt electrolysis, without using metal magnesium, lithium or metal holmium. Therefore, the method greatly shortens the production flow, simplifies the process, decreases the electrolysis temperature, and can reduce the production cost of the alloy.
Owner:HARBIN ENG UNIV
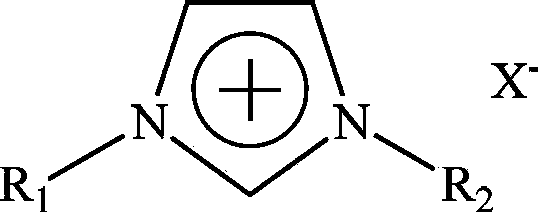
Ionic liquid electroplating solution for preparing bright aluminum coating at low temperature and using method of ionic liquid electroplating solution
The invention provides an ionic liquid electroplating solution for preparing a bright aluminum coating at a low temperature and a using method thereof. The invention relates to the field of green and clean electroplating. The electroplating solution is formed by mixing anhydrous aluminum halide (A), N, N'-dialkyl imidazole halide (B) and an additive (C)-pyridine derivative, wherein an apparent molar ratio of the anhydrous aluminum halide (A) to the N, N'-dialkyl imidazole halide (B) is 1:1-3:1; and the addition amount of the additive (C) is 0.5-15g / L. The operating conditions are that the cathode current density is 0.5-5A / dm<2> under inert gas protection, the plating solution temperature is 25-100 DEG C, the plating time is 10-120minutes, and the stirring speed is 0-500 revolutions per minute. According to the method, the defects that an organic solvent system is narrow in electrochemical window, low in conductivity, high in volatility and simple in combustions and an inorganic fused salt system is high in operating temperature, high in energy consumption and severe in equipment corrosion and environmental pollution and the like are overcome, the obtained coating is low in emissivity and high in purity, the thickness of the coating is easily controlled, and the plating solution can be repeatedly used by quantitatively supplementing additives at fixed time.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com