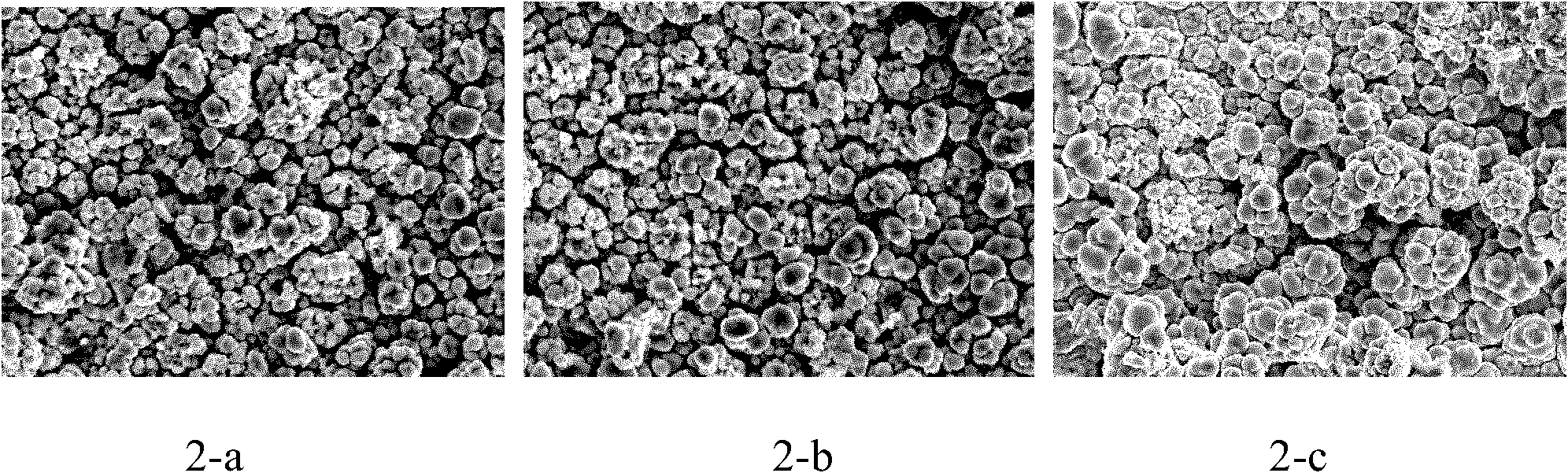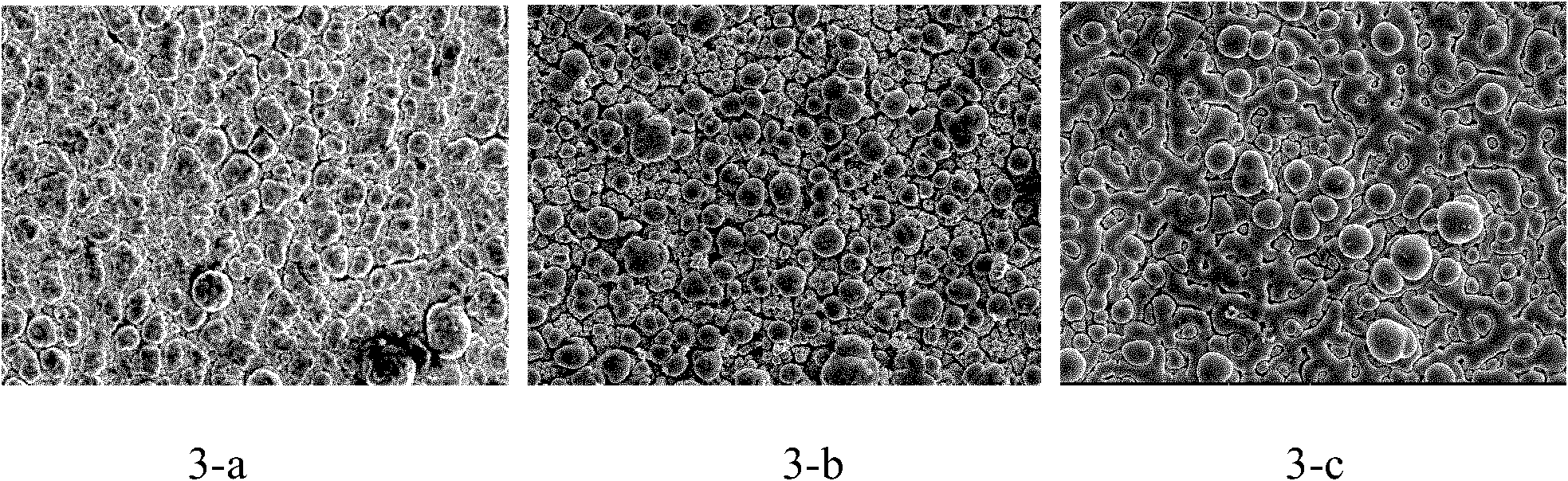Method for performing continuous codeposition on Al-Mn alloy plating layer in molten salt system
An alloy coating and co-deposition technology, applied in the electrolysis process, electrolysis components and other directions, can solve the problem of lack of mass production, and achieve the effects of low production cost, increased probability, and improved metallographic structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
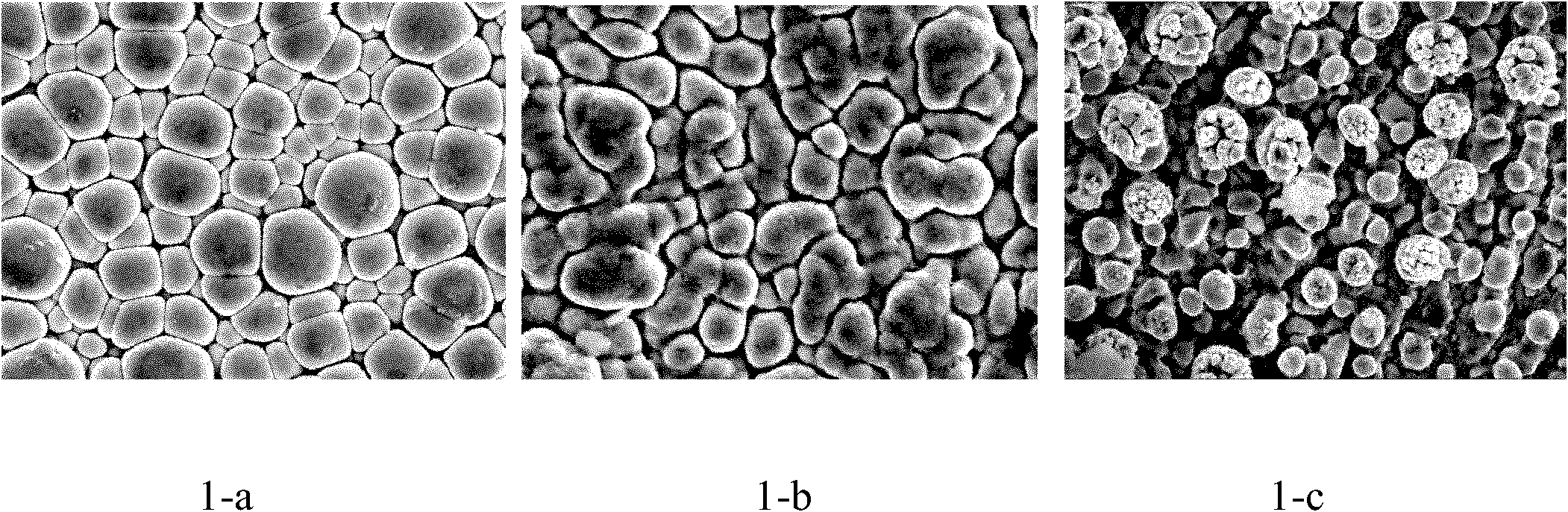
[0036] with NdCl 3 +MnCl 2 +AlCl 3 +NaCl+KCl is the electrolyte system, AlCl 3 150g of NaCl, 19g of NaCl, 25g of KCl; Fe plate is used as cathode, 99% aluminum plate is used as anode, electrolysis temperature is controlled at 180°C, pole distance is 2cm, and current density is controlled at 65mA / cm 2 The tank voltage is controlled at around 2V. For the first plating, add 2.2gMnCl 2 , 0.18gNdCl 3 , After making it dissolve for about 10 minutes, insert the cathode and anode for electroplating, and the electroplating time is 30 minutes. Plating for the second time, add MnCl 2 0.24g, insert the cathode and anode for electroplating, and the electroplating time is 30min. Plating for the third time, add MnCl 2 0.23g, insert the cathode and anode for electroplating, and the electroplating time is 30min. The contents of Al and Mn tend to be similar after three times of plating. NdCl can be added during 3 , but the total amount shall not exceed 0.4% of the total weight.
[0...
Embodiment 2
[0039] with CeCl 3 +MnCl 2 +AlCl 3 +NaCl+KCl is the electrolyte system, AlCl 3 150g of NaCl, 19g of NaCl, 25g of KCl; with steel plate as the cathode and 99% aluminum plate as the anode, the electrolysis temperature is controlled at 180°C, the pole distance is 2cm, and the current density is controlled at 65mA / cm 2 The tank voltage is controlled at around 2V. For the first plating, add 2.1gMnCl 2 , 0.6g CeCl 3 , After making it dissolve for about 10 minutes, insert the cathode and anode for electroplating, and the electroplating time is 30 minutes. Plating for the second time, add MnCl 2 0.24g, insert the cathode and anode for electroplating, and the electroplating time is 30min. Plating for the third time, add MnCl 2 0.237g, insert the cathode and anode for electroplating, and the electroplating time is 30min. The contents of Al and Mn tend to be similar after three times of plating. CeCl can be added during 3 , but the total amount shall not exceed 0.35% of the to...
Embodiment 3
[0042] with LaCl 3 +MnCl 2 +AlCl 3 +NaCl+KCl is the electrolyte system, AlCl 3 150g of NaCl, 19g of NaCl, 25g of KCl; steel plate is used as cathode, 99% aluminum plate is used as anode, electrolysis temperature is controlled at 180°C, pole distance is 2cm, current density is controlled at 69mA / cm 2 The tank voltage is controlled at around 2V. For the first plating, add 2.0gMnCl 2 , 0.25g LaCl 3 , After making it dissolve for about 10 minutes, insert the cathode and anode for electroplating, and the electroplating time is 30 minutes. Plating for the second time, add MnCl 2 0.22g, insert the cathode and anode for electroplating, and the electroplating time is 30min. Plating for the third time, add MnCl 2 0.19g, insert the cathode and anode for electroplating, and the electroplating time is 30min. The contents of Al and Mn tend to be similar after three times of plating. LaCl can be added during 3 , but the total amount shall not exceed 0.38% of the total weight.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com