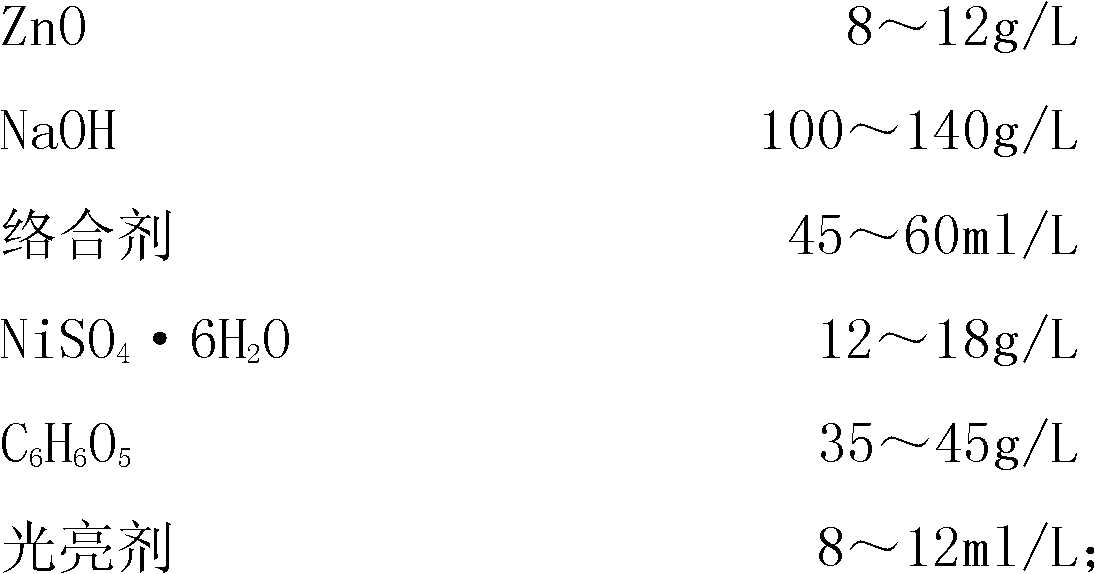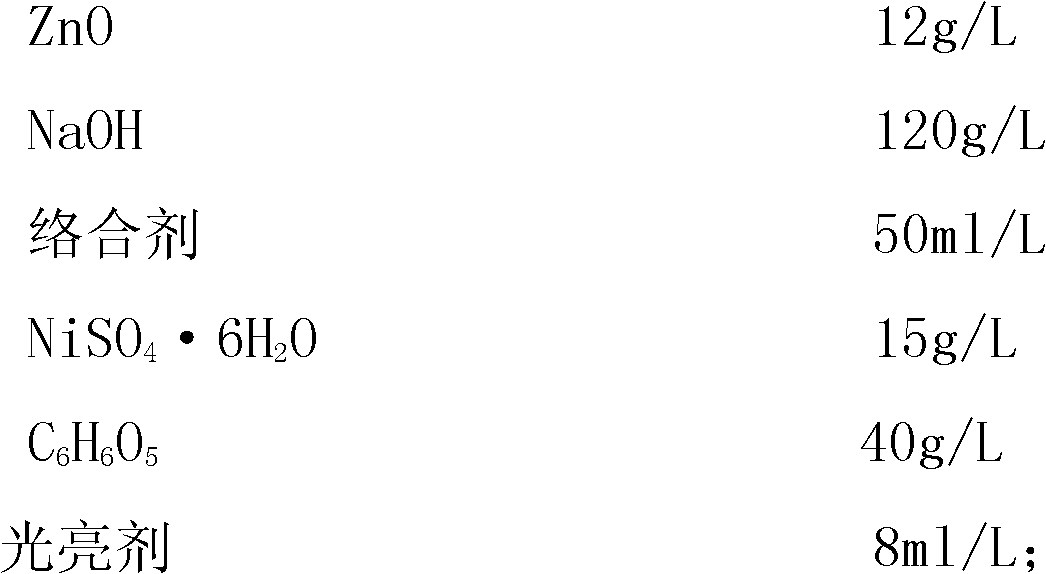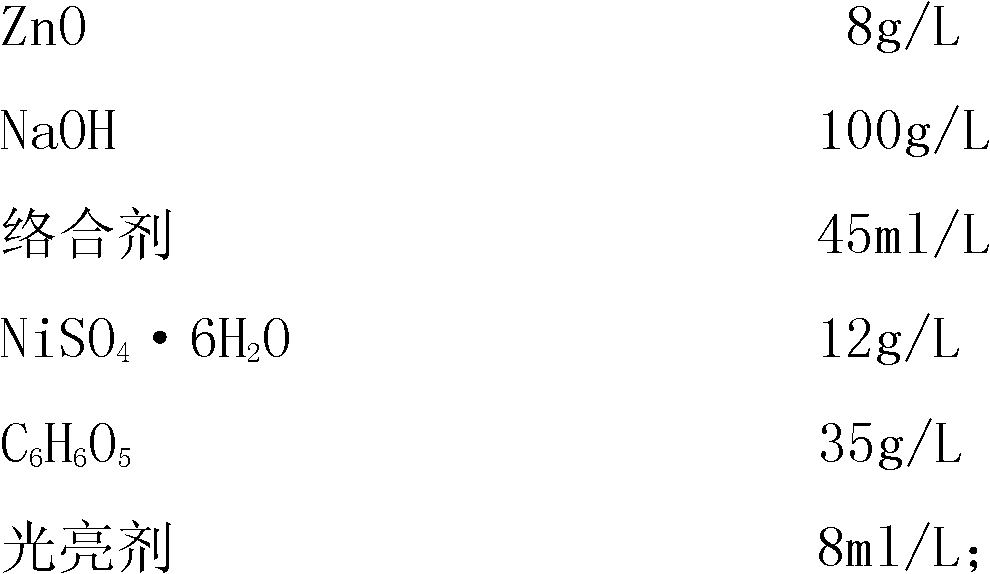Alkali system electroplating bright zinc-nickel alloy process
A technology of nickel alloy and system, applied in the field of bright zinc-nickel alloy electroplating in alkaline system, to achieve the effect of ensuring stability, low brittleness and easy passivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The formulation of the electroplating solution is as follows:
[0038]
[0039] The plating temperature is room temperature, and the cathode current density is 5.0A / dm 2 , Plating time 15 minutes.
[0040] Electroplating solution preparation method of the present invention is as follows, is example with 1000 milliliters:
[0041] (1), in a container with a capacity of 1000 milliliters, put 500 milliliters of deionized water, add 100 grams of NaOH, and stir to dissolve;
[0042] (2), then add 8 grams of ZnO while it is hot, stir, and dissolve it completely; after the temperature drops to room temperature, add 45 milliliters of complexing agent:
[0043] (3), in another 500 ml container, put 250 ml of hot deionized water at 50 degrees, add 35 grams of tartaric acid, 12 grams of NiSO 4 ·6H 2 O stir, dissolve:
[0044] (4) Pour the solution of the above step (3) into the solution of step (2), add 8 ml of brightener, add deionized water to 1000 ml, and stir evenly. ...
Embodiment 2
[0059] The formulation of the electroplating solution is as follows:
[0060]
[0061] The plating temperature is room temperature, and the cathode current density is 1.0A / dm 2 , Plating time 30 minutes.
[0062] Electroplating solution preparation method of the present invention is as follows, is example with 1000 milliliters:
[0063] (1), in a container with a capacity of 1000 milliliters, put 500 milliliters of deionized water, add 120 grams of NaOH, and stir to dissolve;
[0064] (2), then add 10 grams of ZnO while it is hot, stir, and dissolve it completely; after the temperature drops to room temperature, add 50 milliliters of complexing agent:
[0065] (3), in another 500 ml container, put 250 ml of hot deionized water at 50 degrees, add 35 grams of tartaric acid, 12 grams of NiSO 4 ·6H 2 O stir, dissolve:
[0066] (4) Pour the solution of the above step (3) into the solution of step (2), add 8 ml of brightener, add deionized water to 1000 ml, and stir evenly. ...
Embodiment 3
[0081] The formulation of the electroplating solution is as follows:
[0082]
[0083]
[0084] The plating temperature is room temperature, and the cathode current density is 4.0A / dm 2 , Plating time 20 minutes.
[0085] Electroplating solution preparation method of the present invention is as follows, is example with 1000 milliliters:
[0086] (1), in a container with a capacity of 1000 milliliters, put 500 milliliters of deionized water, add 140 grams of NaOH, and stir to dissolve;
[0087] (2), then add 12 grams of ZnO while it is hot, stir, and dissolve it completely; after the temperature drops to room temperature, add 60 milliliters of complexing agent:
[0088] (3), in another 500 ml container, put 300 ml of hot deionized water at 50 degrees, add 45 grams of tartaric acid, 18 grams of NiSO 4 ·6H 2 O stir, dissolve:
[0089] (4) Pour the solution of the above step (3) into the solution of step (2), add 12 milliliters of brightener, add deionized water to 1000...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com