High-current density metal electrolytic deposition device with bottom inlet liquid circulation and realization method thereof
A high current density, electrolytic deposition technology, which is applied in the field of bottom-feeding and circulating high current density electrolytic deposition metal devices, can solve the problems of increasing energy consumption per unit product, decreasing the quality of cathode products, and low production efficiency, and achieving elimination of cathode concentration. Differential polarization effect, improving cathode current density, and improving unit production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
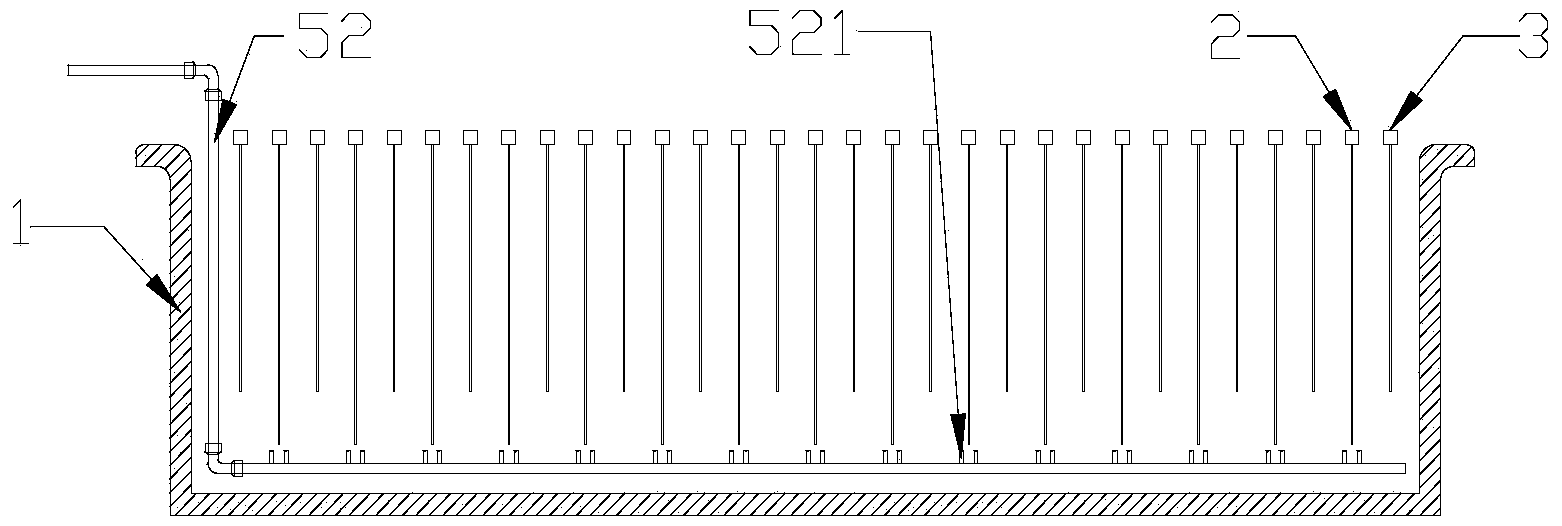
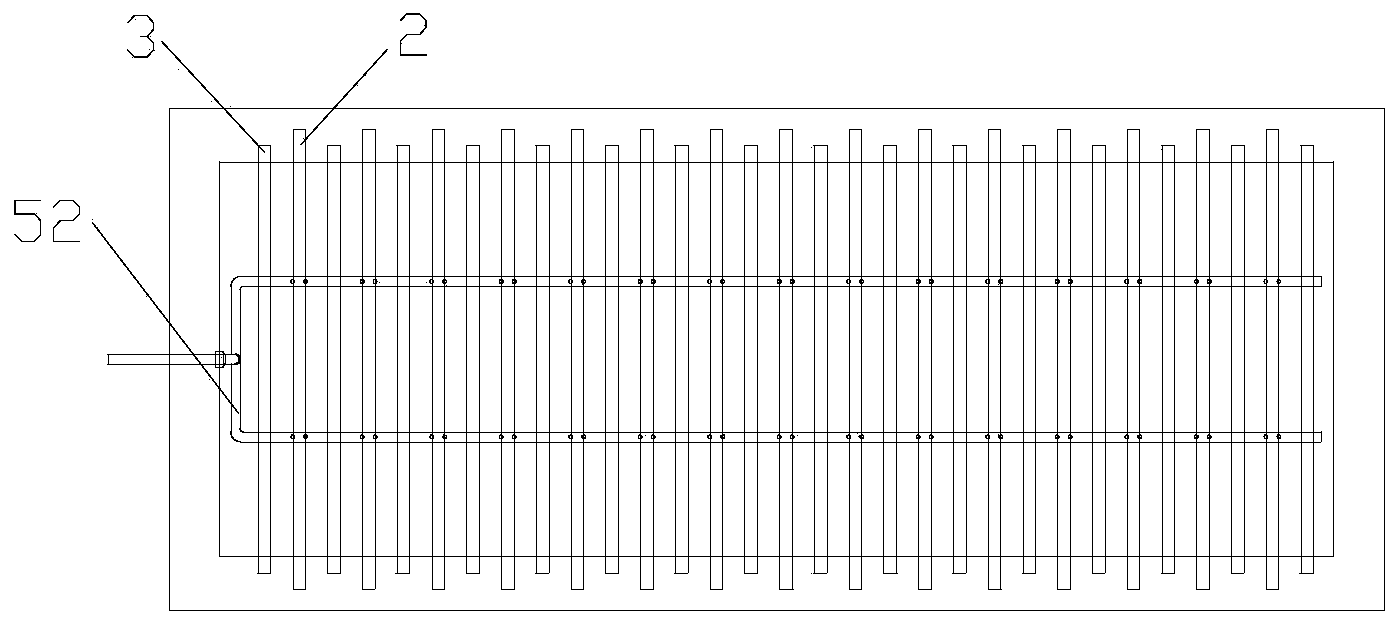
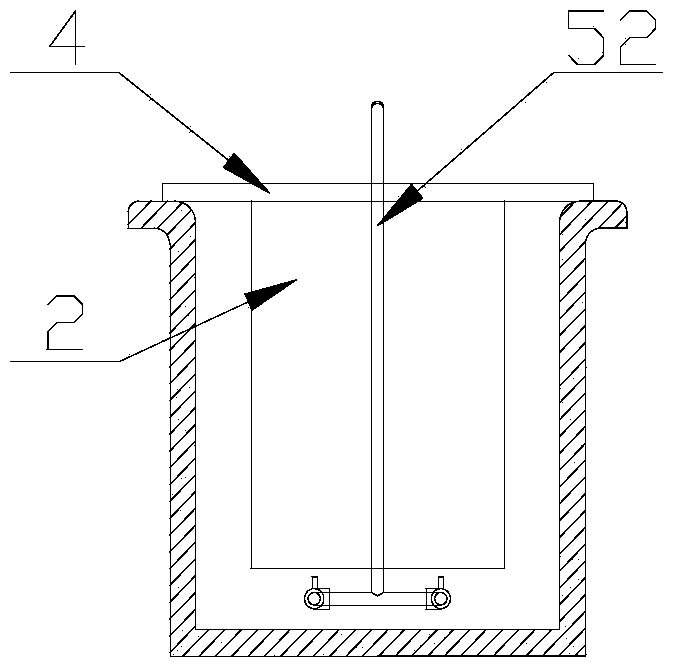
[0025] Warm the copper sulfate solution to 20-60°C, add sulfuric acid to 100-180g / L, turn on the circulation pump 53 in the circulation inflow device 5, so that the catholyte can self-circulate in the electrolytic cell 1, and in each cathode A circulating flow is formed on the surface of the plate 2. When the catholyte self-circulation flow reaches 1500m 3 / Mt, increase the cathode current density to 350A / m 2 , After 4 days of electrolysis, take out the cathode product, the cathode current efficiency reaches 96%, and the output ratio of single-cell cathode product is 200A / m 2 75% increase in time.
Embodiment 2
[0027] Warm the copper sulfate solution to 20-60°C, add sulfuric acid to 100-180g / L, turn on the circulation pump 53 in the circulation inflow device 5, so that the catholyte can self-circulate in the electrolytic cell 1, and in each cathode A circulating flow is formed on the surface of the plate 2. When the catholyte self-circulation flow reaches 1200m 3 / Mt, increase the cathode current density to 300A / m 2 , After 4 days of electrolysis, the cathode product was taken out, the cathode current efficiency reached 98%, and the output ratio of the single-cell cathode product was 200A / m 2 time increased by 50%.
Embodiment 3
[0029] Warm the copper sulfate solution to 20-60°C, add sulfuric acid to 100-180g / L, turn on the circulation pump 53 in the circulation inflow device 5, so that the catholyte can self-circulate in the electrolytic cell 1, and in each cathode A circulating flow is formed on the surface of the plate 2. When the catholyte self-circulation flow reaches 1800m 3 / Mt, increase the cathode current density to 400A / m 2 , after 4 days of electrolysis, take out the cathode product, the cathode current efficiency reaches 95.5%, and the output ratio of single-cell cathode product is 200A / m 2 100% increase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com