Vaccine composition for porcine circovirus and porcine mycoplasma pneumonia and preparation method of vaccine composition
A technology for Mycoplasma suis pneumonia and porcine circovirus, which is applied in the directions of viral antigen components, viruses/phages, biochemical equipment and methods, etc., can solve the problems of heavy workload and time-consuming, and achieves reduction of workload and simplification of epidemic prevention procedures. Avoid the effects of most injection treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
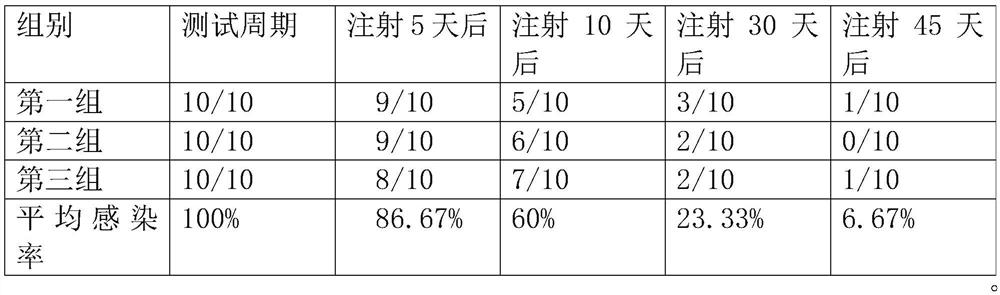
[0034] The proportion of the total amount of the inactivated porcine circovirus type 1 antigen is set to 15%, the proportion of the total amount of the inactivated Mycoplasma hyopneumoniae is set to 25%, and the total amount of acceptable carrier on the pig is The proportion of the amount is set to 42%, the proportion of the total amount of the vaccine adjuvant is set to 10%, and the proportion of the total amount of the serum is set to 8%.
[0035] First extract inactivated porcine circovirus type 1 antigen and inactivated mycoplasma hyopneumoniae;
[0036] adding the prepared inactivated porcine circovirus type 1 antigen and inactivated mycoplasma hyopneumoniae to acceptable carriers on pigs;
[0037] Then add the serum according to the proportion for mixing, and use ultrasonic wave for auxiliary vibration mixing;
[0038] Finally, add the vaccine adjuvant, oscillate and mix, and then perform disinfection treatment. After the treatment is completed, let it stand for 20 minu...
Embodiment 2
[0042] The proportion of the total amount of the inactivated porcine circovirus type 1 antigen is set to 15%, the proportion of the total amount of the inactivated Mycoplasma hyopneumoniae is set to 20%, and the total amount of acceptable carrier on the pig is The proportion of the amount is set to 45%, the proportion of the total amount of the vaccine adjuvant is set to 5%, and the proportion of the total amount of the serum is set to 10%.
[0043] First extract inactivated porcine circovirus type 1 antigen and inactivated mycoplasma hyopneumoniae;
[0044] adding the prepared inactivated porcine circovirus type 1 antigen and inactivated mycoplasma hyopneumoniae to acceptable carriers on pigs;
[0045] Then add the serum according to the proportion for mixing, and use ultrasonic wave for auxiliary vibration mixing;
[0046] Finally, add the vaccine adjuvant, oscillate and mix, and then perform disinfection treatment. After the treatment is completed, let it stand for 20 minu...
Embodiment 3
[0050]The proportion of the total amount of the inactivated porcine circovirus type 1 antigen is set to 10%, the proportion of the total amount of the inactivated Mycoplasma hyopneumoniae is set to 20%, and the total amount of acceptable carrier on the pig is The proportion of the amount is set to 50%, the proportion of the total amount of the vaccine adjuvant is set to 10%, and the proportion of the total amount of the serum is set to 10%.
[0051] First extract inactivated porcine circovirus type 1 antigen and inactivated mycoplasma hyopneumoniae;
[0052] adding the prepared inactivated porcine circovirus type 1 antigen and inactivated mycoplasma hyopneumoniae to acceptable carriers on pigs;
[0053] Then add the serum according to the proportion for mixing, and use ultrasonic wave for auxiliary vibration mixing;
[0054] Finally, add the vaccine adjuvant, oscillate and mix, and then perform disinfection treatment. After the treatment is completed, let it stand for 20 minu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com