Method for preparing and purifying oncolytic virus, and recombinant oncolytic rhabdovirus
An oncolytic rhabdovirus and oncolytic virus technology, applied in the field of biomedicine, can solve the problems of low recovery rate of infectious virus particles, limit the later application of oncolytic virus, increase the production cost of virus vaccine, etc. Effect of inactivation rate control and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
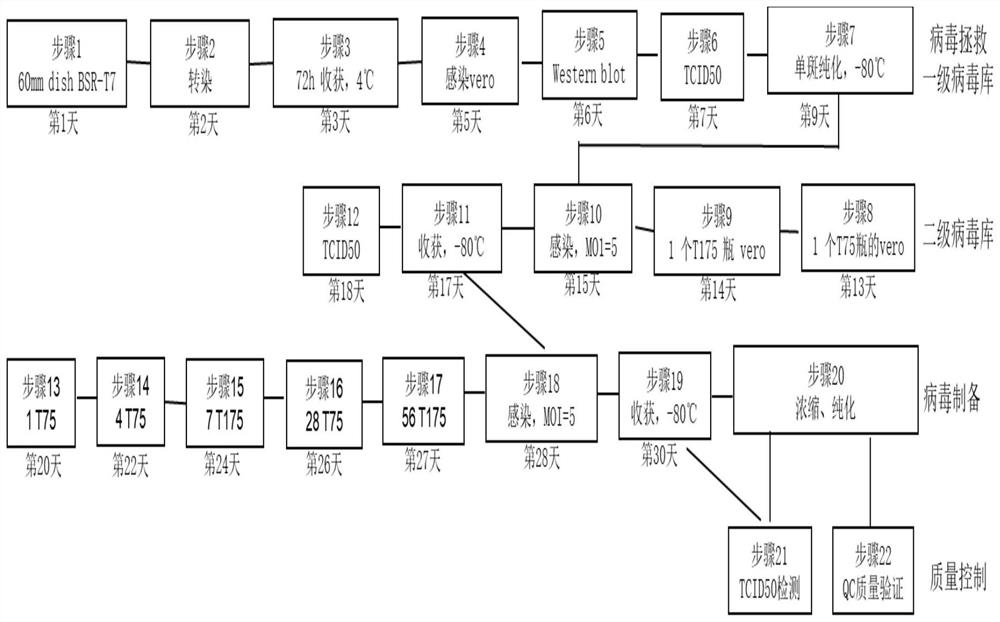
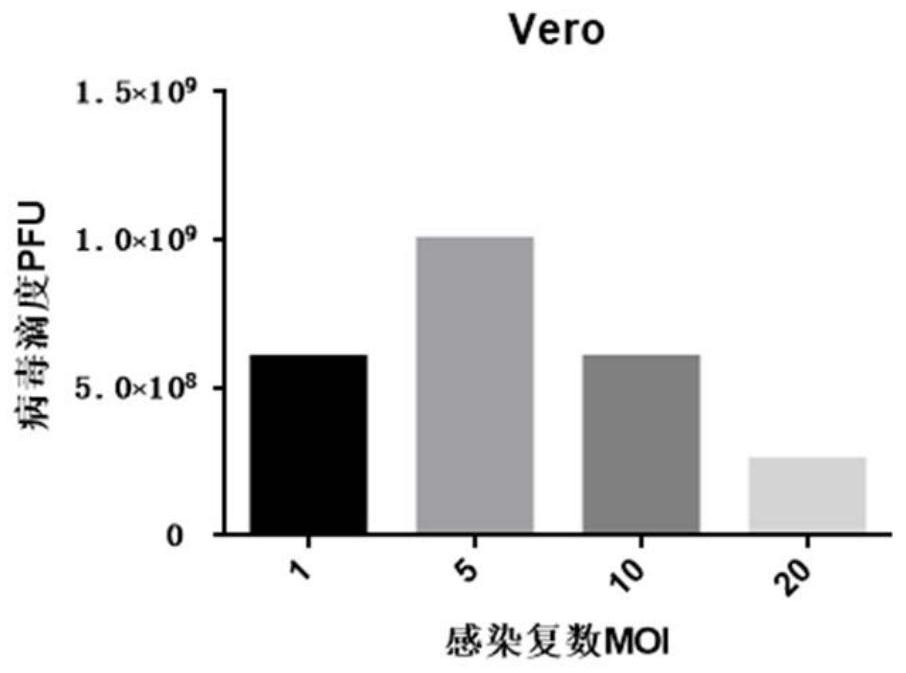
[0077] Example 1 Vero production cells were infected with inoculated doses of different MOI values, and the changes in the total amount of virus titers obtained from production amplification were compared.
[0078] In Vero cells, replace the original complete medium with opti-MEM, then infect Vero cells with VSV virus at MOI=1, 5, 10, and 20, and replace with complete medium after 1h-3h, and wait until the cells are completely lysed After (about 60h), the supernatant was collected to detect the changes in the prepared virus titer (TCID50) under different original virus inoculation (MOI). The overall experimental process refers to figure 1 The specific implementation described in .
[0079] The specific steps of the above experimental process are as follows:
[0080] 1. Add 2 mL of Vero-E6 cell suspension to each well of a 6-well culture plate to make the cell volume reach 4×10 5 Each well, a total of 5 wells, were incubated at 37°C with 5% CO2 by volume for 16 h.
[0081] 2...
Embodiment 2
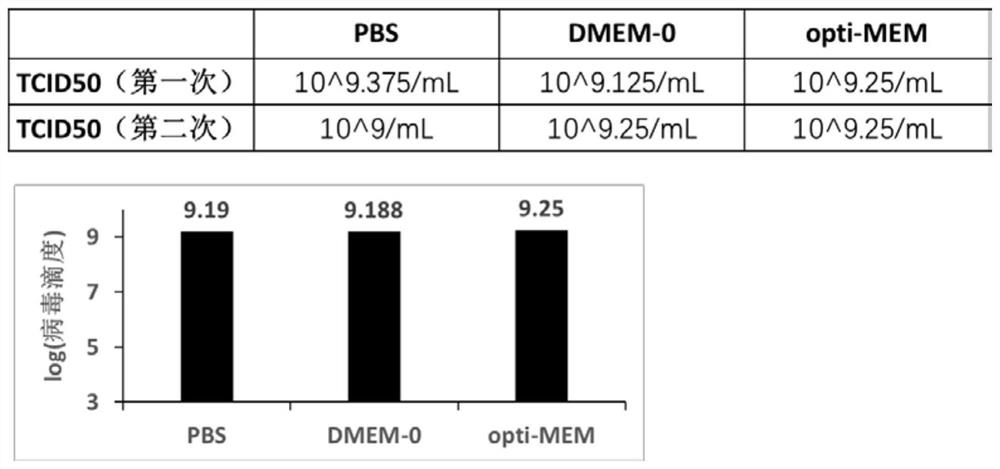
[0089] Example 2 Further, in order to enhance the infectivity of the virus to the cells, PBS, DMEM-0, and opti-MEM were specially selected as the solvent (MOI=5) for the dilution of the U400 virus infection to infect the cells,
[0090] In Vero cells, replace the original medium with PBS, DMEM-0, and opti-MEM respectively, then add VSV virus infection at MOI=5 for 2 hours and replace with complete medium, and collect the supernatant after 48 hours to detect the virus produced by the virus strain The titer (TCID50).
[0091] The specific operation steps of detecting the titer of above-mentioned virus are as follows:
[0092] 1. Add 2 mL of Vero-E6 cell suspension to each well of a 6-well culture plate to make the cell volume reach 4×10 5 cells / well, a total of 4 wells were incubated at 37°C with 5% CO2 by volume for 16 h.
[0093] 2. Take the cells in one of the wells to digest and count them. Dilute the U400 virus to 2 mL with PBS, DMEM-0, and opti-MEM for the cells in the r...
Embodiment 3
[0101] Influence of serum concentration on virus titer in embodiment 3 culture medium
[0102] In the process of traditional virus preparation, it will be found that during the amplification process of some viruses, the serum concentration in the medium will seriously affect the increase of virus titer. In order to solve this problem and further increase the virus titer, optimize the virus Amplification process, in Vero cells, replace the original medium with DMEM-0, then VSV virus infects Vero cells according to MOI=5, and after 2h-3h, use volume percentages of 0%, 1.5%, 3%, and DMEM medium prepared with different concentrations of 6% and 9% FBS was used for virus amplification. After the cells were completely lysed, the supernatant was collected to detect the virus titer (TCID50) under different serum concentrations.
[0103] The concrete steps of detecting the titer of above-mentioned virus are as follows:
[0104] 1. Add 2 mL of Vero-E6 cell suspension to each well of a 6...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap