Luciferase substrates, and preparation method and application thereof
A technology of luciferase and luciferase, which is applied in the field of luciferase substrate and its preparation, can solve the problems that the kinetic isotope effect is still less, and achieve good bioluminescence advantages, long bioluminescence time, Effect of strong bioluminescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
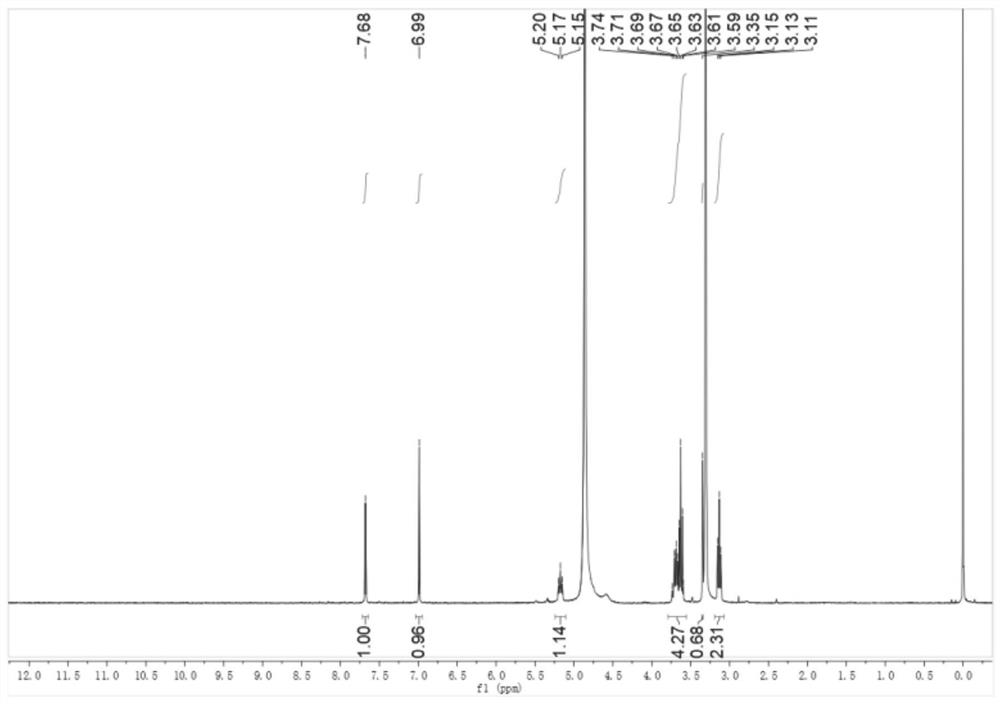
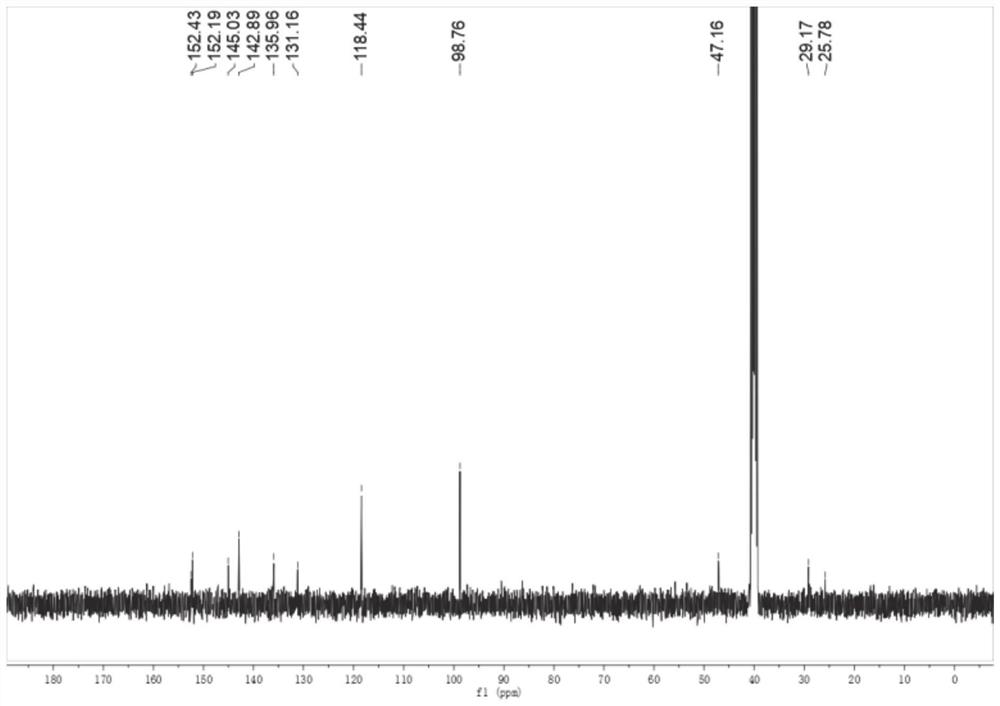
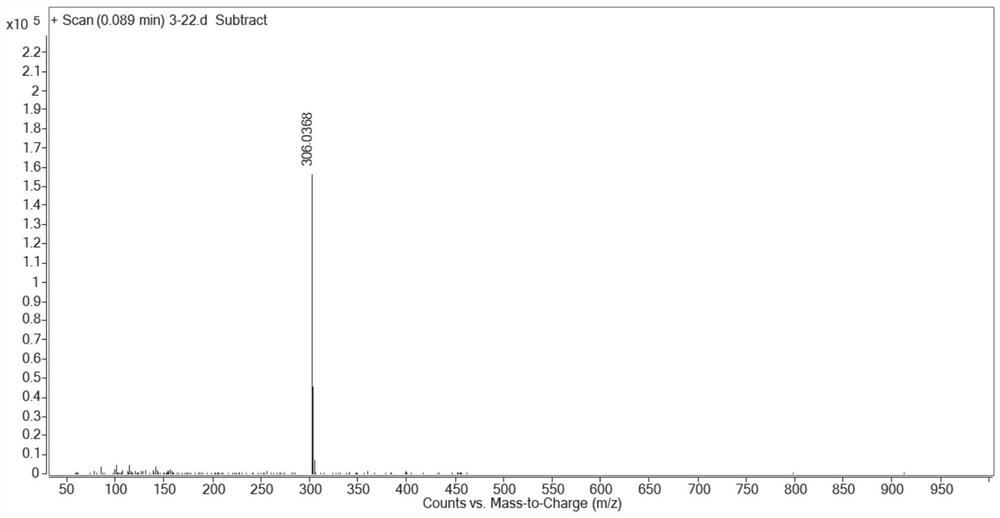
[0134] Embodiment 1: preparation compound cycluc
[0135]
[0136] 2,2,2-trifluoro-1-(5-nitroindolin-1-yl)ethan-1-one (Intermediate 1)
[0137] Add dichloromethane (20mL) to 5-nitroindoline (4g, 24.3mmol) and triethylamine (2.6g, 26.8mmol) and stir, while adding trifluoroacetic anhydride (5.6g, 26.8mmol) dropwise . The mixture was stirred for 45 minutes, then water (50 mL) was added, after stirring for 10 minutes, the mixture was acidified with 5M HCl, the organic layer was washed with brine, dried and concentrated to give Intermediate 1 as a yellow solid 6 g, 94% yield , mp: 136-138°C. 1 H NMR (400MHz, DMSO-d 6 )δ8.23(d, J=10.9Hz, 3H), 4.40(t, J=8.3Hz, 2H), 3.36(d, J=8.2Hz, 2H).
[0138] 1-(5-aminoindolin-1-yl)-2,2,2-trifluoroethan-1-one (Intermediate 2)
[0139] SnCl 2 2H 2 O (14.2 g, 63.1 mmol) was added to a solution of Intermediate 1 (5.5 g, 21.5 mmol) in ethanol (20 mL), and the reaction mixture was heated to reflux at 60° C. for 4 h. After cooling to room t...
Embodiment 2
[0150] Embodiment 2: preparation compound d 2 -cycluc:
[0151]
[0152] indoline-2,3-d 2 (Intermediate 8)
[0153] 1H-indole (20.0g, 170.7mmol) was added into a round-bottomed flask, dissolved with deuterated methanol, added palladium carbon (2.0g, 18.7mmol), and deuterium was bubbled into the reaction under reflux at 40 ° C, and used A pressure of eight atmospheres was applied to the autoclave, monitored by TLC, and the reaction was still incomplete for about 72 hours. Filter to remove palladium carbon, spin dry the reaction solution, add water and extract with ethyl acetate to obtain 11 g of yellow liquid with a yield of 50%, spin dry and directly throw it into the next step.
[0154] 1-(indolin-1-yl-2,3-d 2 )ethan-1-one (Intermediate 9)
[0155] Intermediate 8 (5g, 41.3mmol) was dissolved in glacial acetic acid, and acetyl chloride (19.5mL, 247.6mmol) was added dropwise to the solution at room temperature, and then the reaction solution was moved to 90°C for heat...
Embodiment 3
[0170] Embodiment 3: luciferase substrate cycluc, d 2 Experimental study on metabolism of -cycluc in vitro
[0171] Add compounds cycluc and d in all black 96-well plates 2 -cycluc (20 μM, 50 μL), then add 50 μL of luciferase solution containing 2 mM ATP, start shooting immediately, every 5 min, record the number of photons until the end of 120 min, and use a multifunctional fluorescent microplate reader ( ) to measure the intensity of bioluminescence, each experiment was repeated three times, and statistical calculations were performed with Graphpad.
[0172] Depend on Figure 9 It can be known that firefly luciferase substrates cycluc and d 2 - The bioluminescent intensity of cycluc in vitro decreases with time, and is determined by Figure 9 A-D known d 2 The bioluminescent intensity of -cycluc is much greater than that of cycluc. Within 120min, d 2 - The ratio of bioluminescent intensity between cycluc and cycluc reaches 10.4 times at the minimum and 158.1 times ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com