Amino saccharide compound and preparation method and application thereof
A compound and hydrogen compound technology, which is applied in the field of amino sugar compounds and drugs for the treatment of type 2 diabetes, can solve the problems of unsimplified steps, cumbersome operation, and high cost, so as to save purification work, avoid side reactions, and be easy to operate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
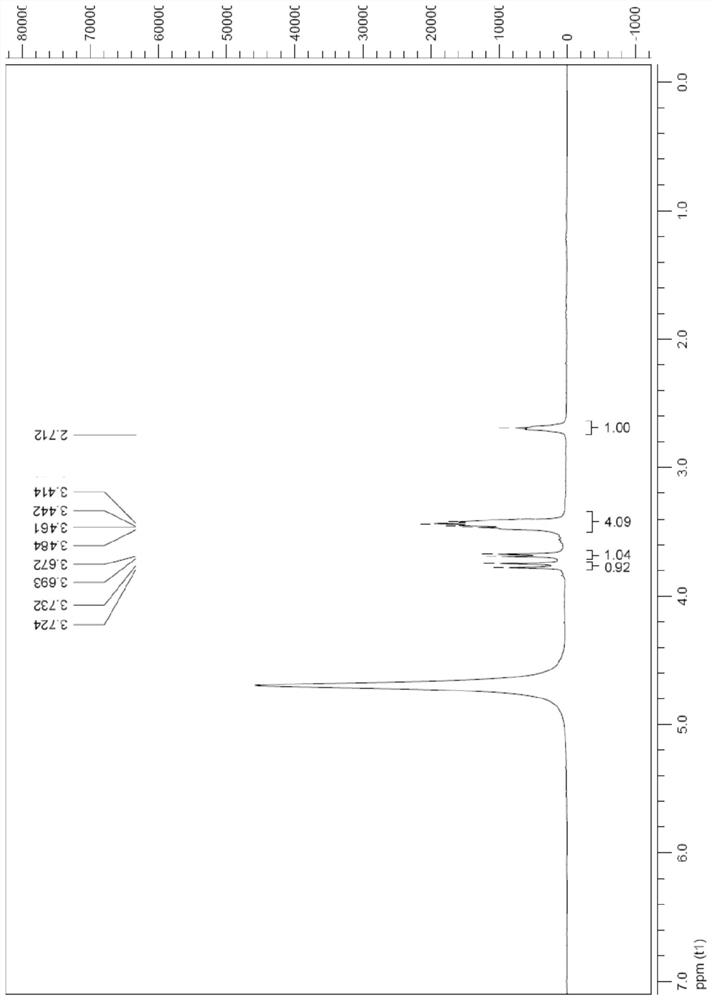
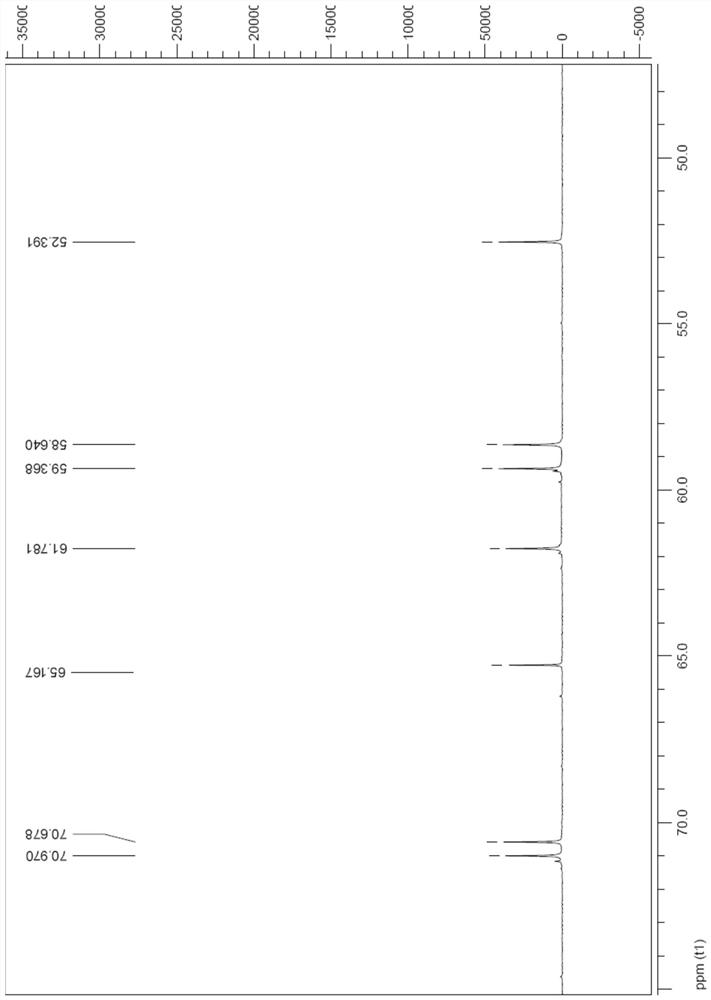
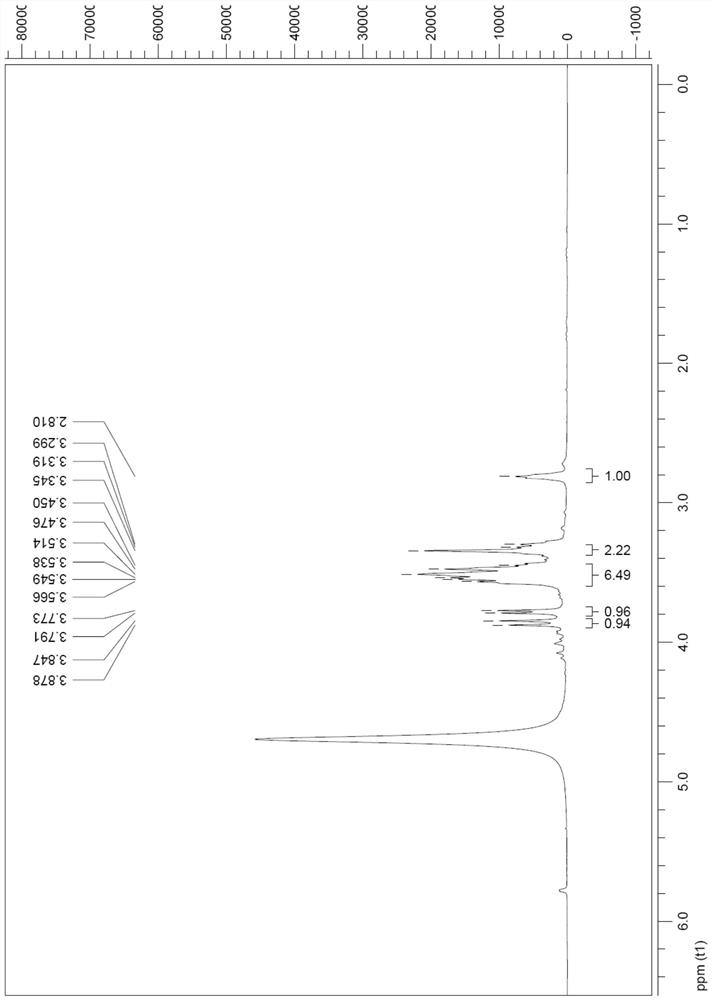
[0072] The preparation of compound shown in formula Ia
[0073] Add the raw material IIa (4.0g, 0.023mol) to DMF (18mL), cool to 0°C, add p-toluenesulfonic acid (4.3g, 0.025mol), after the addition is complete, naturally rise to room temperature, stir for 5h, add m-chloro Peroxybenzoic acid (m-CPBA) (6.0 g, 0.035 mol) was reacted at 40° C. for 48 h. Cool to 0°C, add Me 2 S (2.6mL, 0.035mol), after stirring for 30min, add Na 2 CO 3 (5.0g, 47.2mmol) and MgSO 4 (5.0g, 41.5mmol), stirred at room temperature for 5h, filtered with suction, washed the filter cake with methanol (10mL), evaporated the solvent under reduced pressure, then separated with UBK resin, eluted with water and 2N ammonia water in sequence, collected the eluate and concentrated , to obtain compound Ia2.9g, yield 68%.
Embodiment 2
[0075] The preparation of compound shown in formula Ia
[0076] Add raw material IIa (4.0g, 0.023mol) to methanol (30mL), cool to 0°C, add p-chlorobenzenesulfonic acid (4.8g, 0.025mol), after addition, stir at room temperature for 3h, add methyltrifluoromethane Ketone peroxide (TFDO) (5.9g, 0.046mol) was reacted at room temperature for 48h. Cool to 0°C, add Me 2 S (3.4mL, 0.046mol), after stirring for 30min, add Na 2 CO 3 (5.0g, 47.2mmol) and MgSO 4 (5.0g, 41.5mmol), stirred at room temperature for 5h, filtered with suction, washed the filter cake with methanol (10mL), evaporated the solvent under reduced pressure, then separated with UBK resin, eluted with water and 2N ammonia water in sequence, collected the eluate and concentrated , to obtain compound Ia 2.7g, yield 62%.
Embodiment 3
[0078] The preparation of compound shown in formula Ia
[0079] Add raw material IIa (4.0g, 0.023mol) to methanol (30mL), cool to 0°C, add p-toluenesulfonic acid (4.3g, 0.025mol), after the addition is complete, naturally warm to room temperature, stir for 5h, add dimethyl Ketone peroxide (DMDO) (3.4g, 0.046mol) was reacted at 50°C for 24h. Cool to 0°C, add Me 2 S (3.4mL, 0.046mol), after stirring for 1h, add Na 2 CO 3 (5.0g, 47.2mmol) and MgSO 4 (5.0g, 41.5mmol), stirred at room temperature for 5h, filtered with suction, washed the filter cake with methanol (10mL), evaporated the solvent under reduced pressure, then separated with UBK resin, eluted with water and 2N ammonia water in sequence, collected the eluate and concentrated , to obtain 3.0 g of compound Ia with a yield of 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com