Liquid biopsy kit for diagnosis of lymph node metastasis in papillary thyroid carcinoma
A technique for lymph node metastasis and papillary carcinoma, which is used in the determination/inspection of microorganisms, biological tests, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
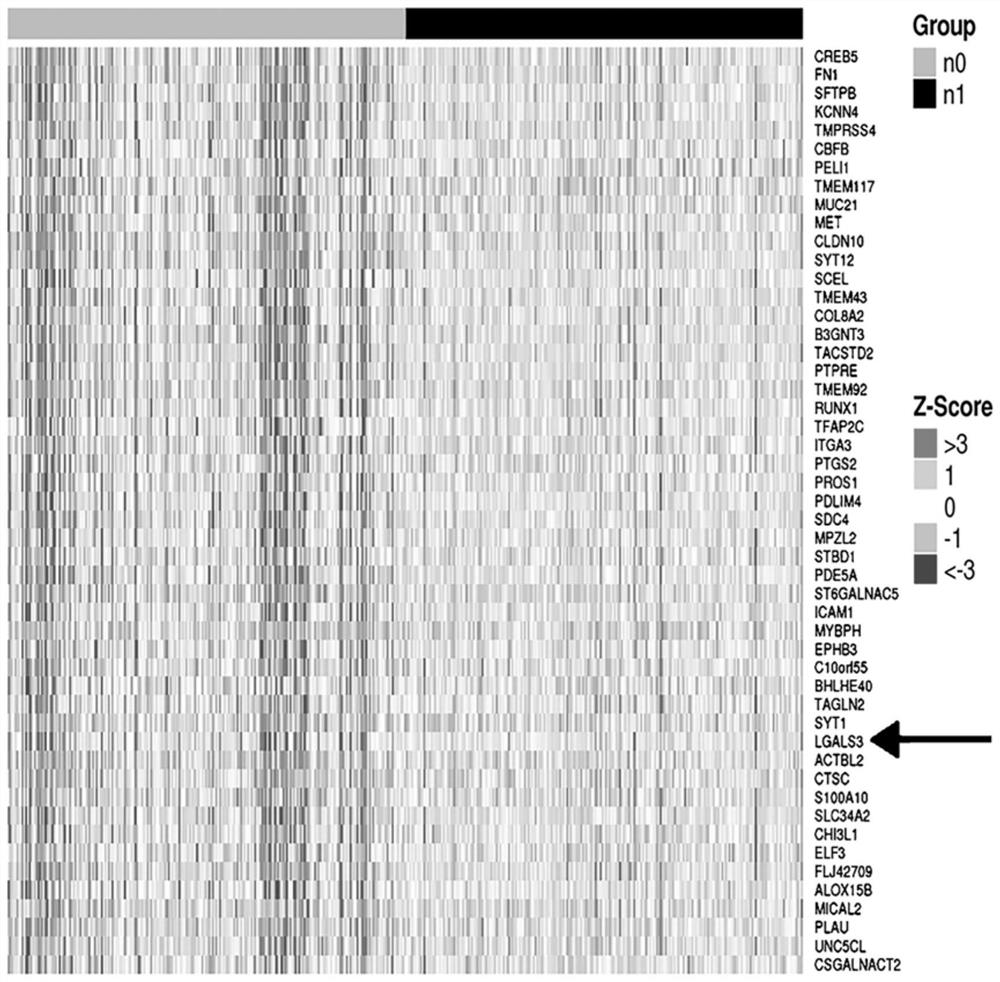
[0038] Example 1: Establishment of a combined detection scheme of hsa-circ-UMAD1 combined with galectin-3 (1) Detection of galectin-3 in peripheral blood circulation and lymph node metastasis in patients with papillary thyroid carcinoma using an enzyme-linked immunosorbent assay kit
[0039] Galectin-3 was detected using an enzyme-linked immunosorbent assay kit (Bio-Swamp, Beijing, China).
[0040] Reagents: enzyme-labeled coated plate; Galectin-3 standard; Standard dilution; Biotin-labeled Galectin-3 antibody; Chromogenic solution A; Chromogenic solution B; Stop solution; Enzyme labeling reagent; Concentration detergent.
[0041] method:
[0042] 1. Standard dilution: Prepare 6 small test tubes, number them in sequence, first add 100 μl of standard dilution solution to each small test tube, and then add 100 μl of the original concentration standard to a numbered test tube, fully Mix well; then add 100 μl from the test tube to the second test tube and mix thoroughly; then ...
Embodiment 2
[0076] Example 2: Implementation of the combined detection scheme of hsa-circ-UMAD1 combined with galectin-3
[0077] Research Proposal:
[0078] 1. Take fresh peripheral blood samples. Plasma samples were collected in EDTA tubes and centrifuged at 1500 rpm. Within 2 hours, one of the plasma samples was immediately used for RNA extraction without freezing, and the other was directly subjected to ELISA (with or without freezing).
[0079] 2. Use two kits in combination, one is a real-time quantitative kit, and the other is an ELISA kit.
[0080] specific method:
[0081] 2.1 Detection of hsa-circ-UMAD1 using a real-time quantitative kit (Invitrogen USA)
[0082] 2.1.1 Reagents: lysis solution (main component Trizol); purification column for total RNA extraction (Qiagen miRNeasy Mini Kit Germany); RNase R; short fragment RNA purification column (Qiagen RNeasy MiniElute Purification Kit Germany); dNTP; random primers; reverse Transcriptase M-MLV; DTT; PCR mix reagent (PowerU...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com