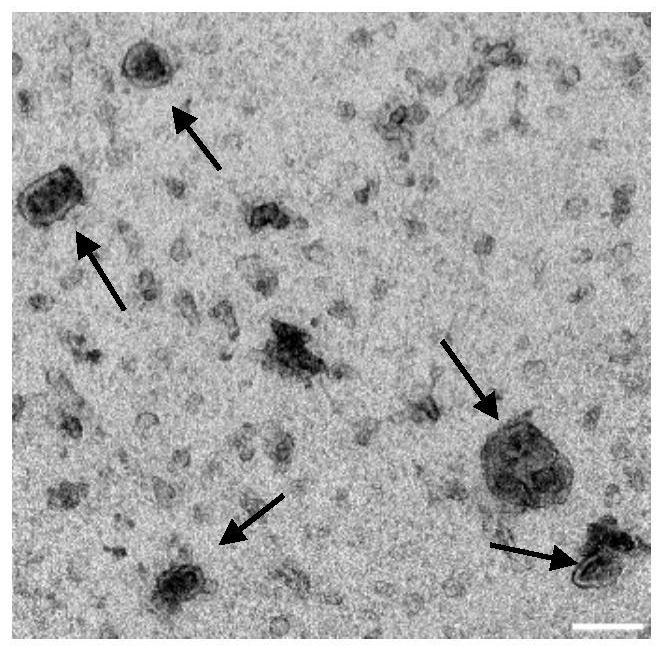
Application of miR-493-5p detection reagent in preparation of esophageal cancer detection kit and esophageal cancer detection kit
A detection kit and detection reagent technology, applied in the fields of biotechnology and medicine, can solve the problems of inability to diagnose esophageal cancer, low specificity, and large patient trauma, and achieve high clinical use value and promotion value, good specificity, highly specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
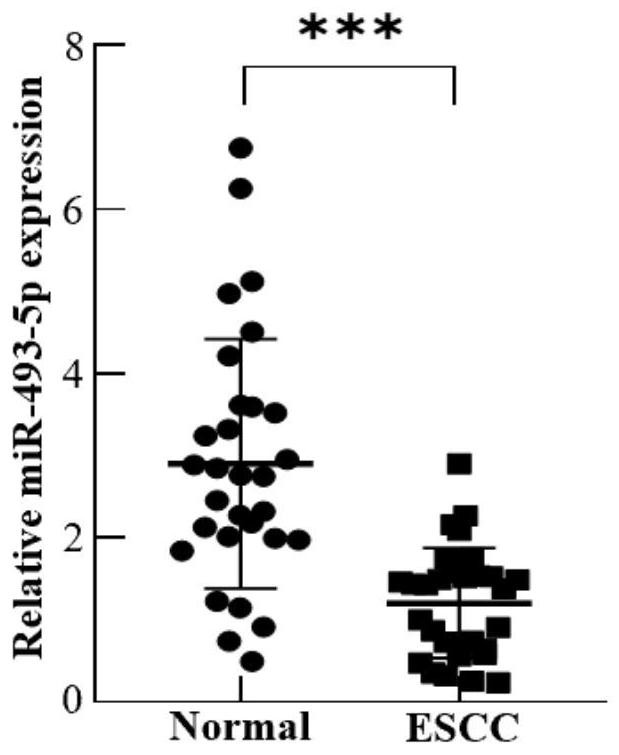
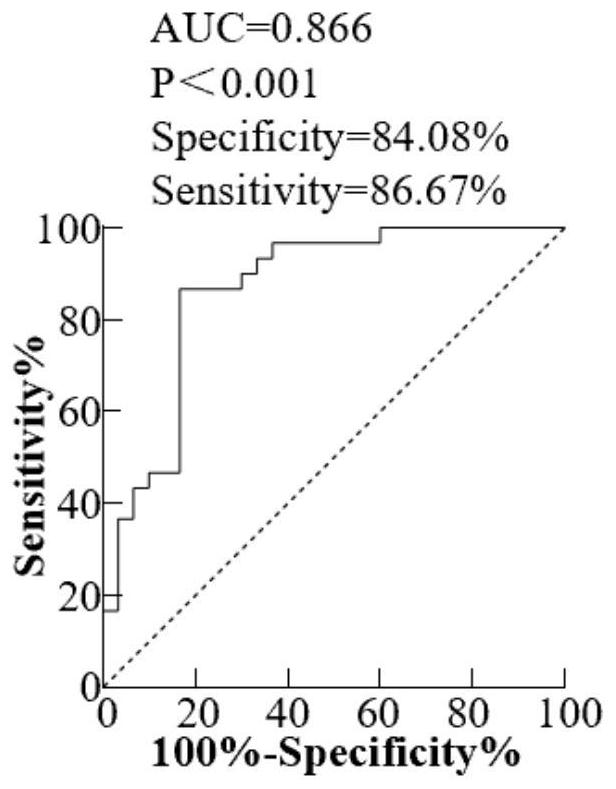
[0033] Example 1, the expression level of miR-493-5p in plasma exosomes of patients with early esophageal cancer
[0034] 1. Research objects: 30 patients with early esophageal cancer (according to postoperative pathological TNM stage: T1N0M0) admitted to the Second Hospital of Shandong University from August 2019 to June 2020 were included as the experimental group, and 30 healthy volunteers were included as the experimental group. control group. None of the included patients with esophageal cancer received neoadjuvant chemoradiotherapy before operation.
[0035] 2. Plasma collection: Collect about 3mL of peripheral blood from patients with early esophageal cancer before operation, place them in a vacuum tube containing EDTA anticoagulant and mix them upside down, centrifuge at 1900g, 4°C for 10min, and collect the supernatant; Centrifuge again at ℃ for 15 minutes and carefully pipette the supernatant into a cryopreservation tube, freeze it in liquid nitrogen and store it in...
Embodiment 2
[0060] Example 2. Differences in the expression level of miR-493-5p between patients with and without lymph node metastasis of esophageal cancer
[0061] The qPCR method was used to quantify the level of miR-493-5p in the preoperative plasma exosomes of 30 groups of esophageal cancer patients with lymph node metastasis and 30 groups of esophageal cancer patients without lymph node metastasis detected by postoperative pathology, so as to determine the level of plasma exosomes. Whether miR-493-5p can be used as a biomarker to judge whether there is lymph node metastasis in patients with esophageal cancer.
[0062] Collect the preoperative plasma of esophageal cancer patients with and without lymph node metastasis, separate plasma exosomes, extract the total RNA of plasma exosomes, measure the concentration of the extracted total RNA, and use the tailing method to reverse transcribe to obtain cDNA, and the obtained cDNA Real-time fluorescent quantitative PCR was carried out, all ...
Embodiment 3
[0066] Example 3. Differences in miR-493-5p expression levels in esophageal cancer plasma exosomes before and after surgery
[0067] The plasma of patients with esophageal cancer before operation and 7 days after operation was collected, plasma exosomes were separated, total RNA of plasma exosomes was extracted, the concentration of the extracted total RNA was measured, cDNA was obtained by reverse transcription by tailing method, and the obtained cDNA Real-time fluorescent quantitative PCR was carried out, all of which were carried out according to the operation method in Example 1.
[0068] Real-time fluorescence quantitative results such as Figure 6 As shown, by comparing the expression of miR-493-5p in plasma exosomes of patients with esophageal cancer before and after surgery, it was found that the expression level of miR-493-5p in plasma exosomes of patients with esophageal cancer after surgery was significantly higher than that before surgery. High (p<0.001). The kit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com